NPs Basic Information
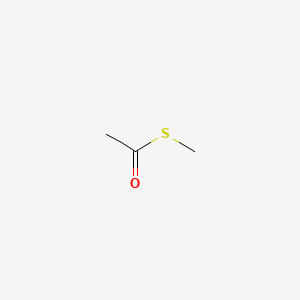
|
Name |
S-Methyl thioacetate
|
| Molecular Formula | C3H6OS | |
| IUPAC Name* |
S-methyl ethanethioate
|
|
| SMILES |
CC(=O)SC
|
|
| InChI |
InChI=1S/C3H6OS/c1-3(4)5-2/h1-2H3
|
|
| InChIKey |
OATSQCXMYKYFQO-UHFFFAOYSA-N
|
|
| Synonyms |
S-Methyl thioacetate; 1534-08-3; S-Methyl ethanethioate; Ethanethioic acid, S-methyl ester; Methylthioacetate; Methanethiol acetate; Thioacetic acid S-methyl ester; S-METHYLTHIOACETATE; Methyl thiolacetate; methyl ethanethioate; CH3C(O)SCH3; Acetic acid, thio-, S-methyl ester; PF2D4MWX79; 1-(methylsulfanyl)ethan-1-one; Ethanethioic acid, methyl ester; UNII-PF2D4MWX79; AcSMe; EINECS 216-252-1; S-Methyl ethanethioate #; S-METHYLTHIOACETIC ACID; DTXSID3073264; FEMA NO. 3876; CHEBI:51280; FEMA 3876; S-Methyl thioacetate, AldrichCPR; S-METHYL THIOACETATE [FHFI]; ZINC2004049; MFCD00014989; AKOS006229807; CS-0154991; FT-0659567; M2286; S-Methyl thioacetate, natural, >=96%, FG; D82067; EN300-7016730; Q-100182; Q27104783
|
|
| CAS | 1534-08-3 | |
| PubChem CID | 73750 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 90.15 | ALogp: | 0.7 |
| HBD: | 0 | HBA: | 2 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 42.4 | Aromatic Rings: | 0 |
| Heavy Atoms: | 5 | QED Weighted: | 0.446 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.549 | MDCK Permeability: | 0.00001740 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.008 | 20% Bioavailability (F20%): | 0.003 |
| 30% Bioavailability (F30%): | 0.003 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.992 | Plasma Protein Binding (PPB): | 36.53% |
| Volume Distribution (VD): | 0.764 | Fu: | 76.56% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.645 | CYP1A2-substrate: | 0.915 |
| CYP2C19-inhibitor: | 0.111 | CYP2C19-substrate: | 0.859 |
| CYP2C9-inhibitor: | 0.01 | CYP2C9-substrate: | 0.442 |
| CYP2D6-inhibitor: | 0.012 | CYP2D6-substrate: | 0.484 |
| CYP3A4-inhibitor: | 0.006 | CYP3A4-substrate: | 0.326 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 10.321 | Half-life (T1/2): | 0.851 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.022 | Human Hepatotoxicity (H-HT): | 0.121 |
| Drug-inuced Liver Injury (DILI): | 0.527 | AMES Toxicity: | 0.017 |
| Rat Oral Acute Toxicity: | 0.044 | Maximum Recommended Daily Dose: | 0.431 |
| Skin Sensitization: | 0.864 | Carcinogencity: | 0.779 |
| Eye Corrosion: | 0.977 | Eye Irritation: | 0.992 |
| Respiratory Toxicity: | 0.028 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000656 | 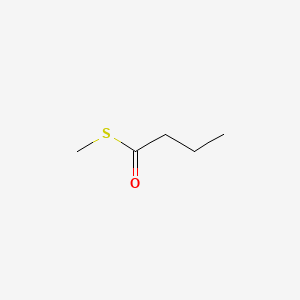 |
0.381 | D04CRL |  |
0.357 | ||
| ENC000008 | 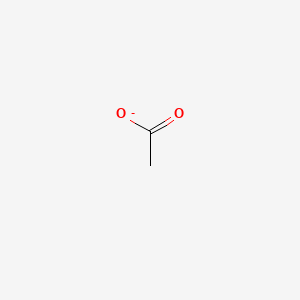 |
0.357 | D0Z4UY | 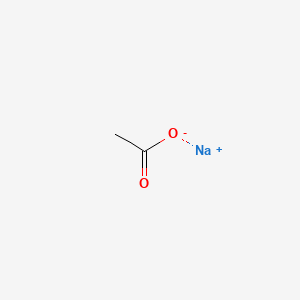 |
0.333 | ||
| ENC000009 | 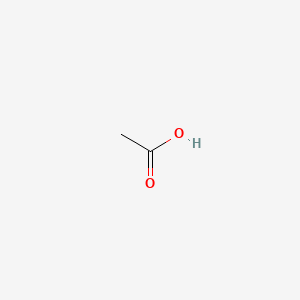 |
0.357 | D0C1PY | 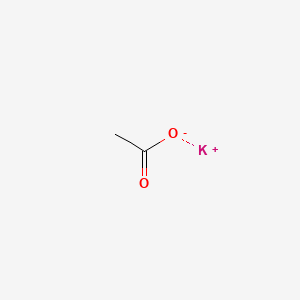 |
0.333 | ||
| ENC000010 | 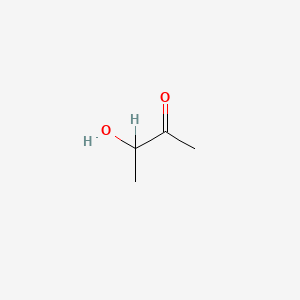 |
0.333 | D0R9BG | 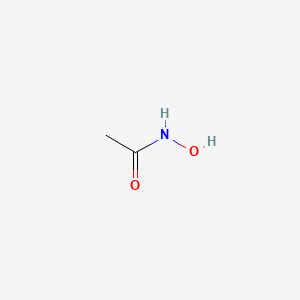 |
0.294 | ||
| ENC000312 | 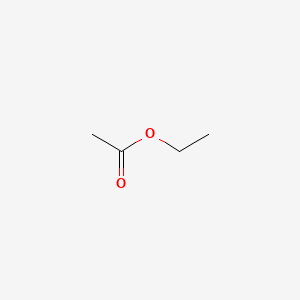 |
0.300 | D0F1GS | 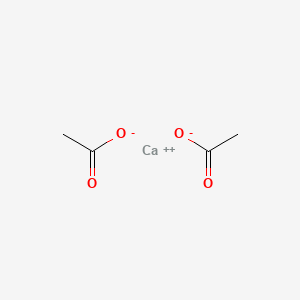 |
0.273 | ||
| ENC000288 | 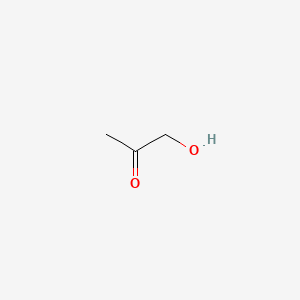 |
0.294 | D0Z4NI | 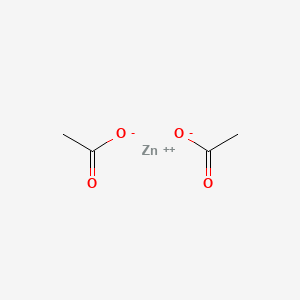 |
0.273 | ||
| ENC000313 | 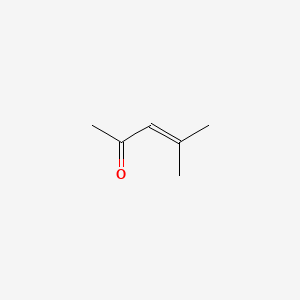 |
0.273 | D0G4JI | 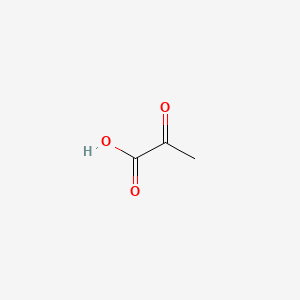 |
0.263 | ||
| ENC000532 | 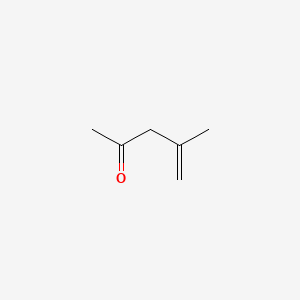 |
0.273 | D0ZK8H | 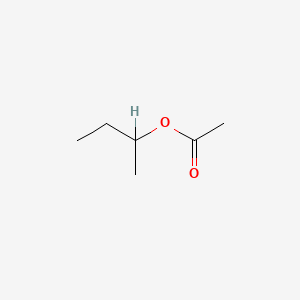 |
0.240 | ||
| ENC000237 | 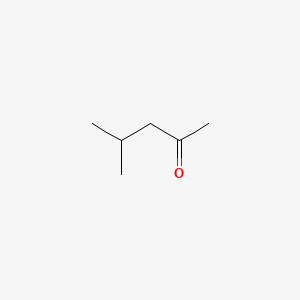 |
0.273 | D0FM2P | 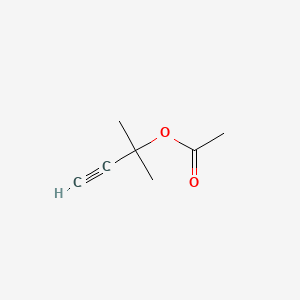 |
0.222 | ||
| ENC000418 | 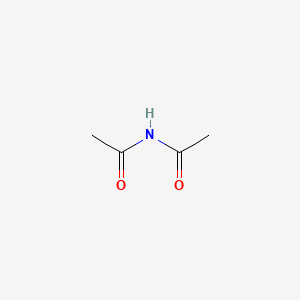 |
0.273 | D0Q9HF | 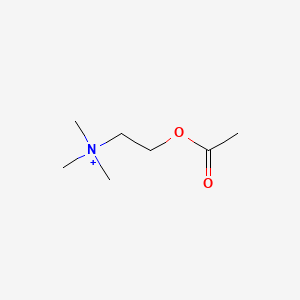 |
0.200 | ||