NPs Basic Information
|
Name |
Methyl isobutyl ketone
|
| Molecular Formula | C6H12O | |
| IUPAC Name* |
4-methylpentan-2-one
|
|
| SMILES |
CC(C)CC(=O)C
|
|
| InChI |
InChI=1S/C6H12O/c1-5(2)4-6(3)7/h5H,4H2,1-3H3
|
|
| InChIKey |
NTIZESTWPVYFNL-UHFFFAOYSA-N
|
|
| Synonyms |
4-Methyl-2-pentanone; METHYL ISOBUTYL KETONE; 4-Methylpentan-2-one; 108-10-1; Isopropylacetone; Isobutyl methyl ketone; MIBK; 2-Pentanone, 4-methyl-; Hexone; 2-Methyl-4-pentanone; 4-Methyl-2-oxopentane; Methylisobutylketon; Isohexanone; Hexon; Shell mibk; Metilisobutilchetone; Metyloizobutyloketon; 2-Methylpropyl methyl ketone; Isobutyl-methylketon; Methyl-isobutyl-cetone; 4-Methyl-2-pentanon; 4-Metilpentan-2-one; Isopropyl acetone; Ketone, isobutyl methyl; 4-Methyl-pentan-2-on; 4-Methyl-pentan-2-one; Rcra waste number U161; FEMA No. 2731; NSC 5712; methyl isobutylketone; METHYL ISO-BUTYL KETONE; 2-Methyl-4-pentanal; Methyl I-butyl ketone; ethyl iso-butyl ketone; MFCD00008938; U5T7B88CNP; Methyl isobutyl ketone [NF]; CHEBI:82344; NSC-5712; Hexon [Czech]; Methyl isobutyl ketone (NF); MIK; methylisobutyl ketone; 4-Methyl-2-pentanone, >=99%; Caswell No. 574AA; FEMA Number 2731; methylisobutylketone; Isobutyl-methylketon [Czech]; Metyloizobutyloketon [Polish]; isobutylmethyl ketone; Metilisobutilchetone [Italian]; 4-Methyl-2-pentanon [Czech]; CCRIS 2052; HSDB 148; Methyl-isobutyl-cetone [French]; 4-Metilpentan-2-one [Italian]; 4-Methyl-2-pentanone (natural); 2-Pentanone,4-methyl-; Methylisobutylketon [Dutch, German]; EINECS 203-550-1; UN1245; RCRA waste no. U161; UNII-U5T7B88CNP; EPA Pesticide Chemical Code 044105; BRN 0605399; 4-Methyl-pentan-2-on [Dutch, German]; AI3-01229; methylisobutyketone; isobutylmethylketone; methylisobutlyketone; i-BuCOMe; methylisobutyl keton; methylisobutyl-keton; Methylpentan-2-one; iso-butylmethylketone; methyl-isobutylketone; 4-methyl-2pentanone; methy isobutyl ketone; methyl isobutyl keton; methyl iso-butylketone; methyl-iso-butylketone; methyl-isobutyl ketone; 4-methylpentane-2-one; iso-C4H9COCH3; Methyl-2-pentanon,4-; 4-methyl- 2-pentanone; MIBK [INCI]; DSSTox_CID_1889; EC 203-550-1; DSSTox_RID_76387; DSSTox_GSID_21889; SCHEMBL15458; ISOPROPYLACETONE [MI]; 4-01-00-03305 (Beilstein Handbook Reference); 4-Methyl-2-pentanone(MIBK); CHEMBL285323; DTXSID5021889; SCHEMBL13341539; NSC5712; Methyl isobutyl ketone, ACS grade; AMY11098; ZINC1482107; 4-Methyl-2-pentanone, HPLC Grade; Methylisobutylketon(DUTCH, GERMAN); Tox21_201108; WLN: 1Y1 & 1V1; 4-METHYL-2-PENTANONE [FCC]; LMFA12000033; METHYLISOBUTYLKETONE [USP-RS]; METHYL ISOBUTYL KETONE [HSDB]; METHYL ISOBUTYL KETONE [IARC]; 4-METHYL-2-PENTANONE [FHFI]; AKOS000118793; 4-Methyl-2-pentanone, AR, >=99%; 4-Methyl-2-pentanone, LR, >=99%; METHYL ISOBUTYL KETONE [MART.]; UN 1245; 4-Methyl-2-pentanone, >=99%, FCC; METHYL ISOBUTYL KETONE [USP-RS]; NCGC00091475-01; NCGC00091475-02; NCGC00258660-01; 4-Methyl-pentan-2-on(DUTCH, GERMAN); BP-13453; CAS-108-10-1; Methyl Isobutyl Ketone Reagent Grade ACS; 4-Methyl-2-pentanone, analytical standard; FT-0628744; M0389; 4-Methyl-2-pentanone, technical grade, 95%; 4-Methyl-2-pentanone, for HPLC, >=99.5%; C19263; D04989; 4-Methyl-2-pentanone, ACS reagent, >=98.5%; A801806; Q418104; 4-Methyl-2-pentanone, SAJ first grade, >=99.0%; J-515799; Methyl isobutyl ketone, p.a., ACS reagent, 98.5%; Q-200495; 2-PENTANONE,4-METHYL METHYL,ISOBUTYL,KETONE; 4-Methyl-2-pentanone, JIS special grade, >=99.5%; Methyl isobutyl ketone [UN1245] [Flammable liquid]; F1908-0087; 4-Methyl-2-pentanone, puriss. p.a., ACS reagent, >=99.0% (GC); 4-Methyl-2-pentanone, puriss., ACS reagent, reag. Ph. Eur., 99.0%; 4-Methyl-2-pentanone, suitable for atomic absorption spectrometry, >=99.5%; ALFA-[(PHENYLMETHOXY)CARBONYL]OXY-1-PIPERIDINEACETICACIDMETHYLESTER; Methyl isobutyl ketone, United States Pharmacopeia (USP) Reference Standard; Methyl Isobutyl Ketone, Pharmaceutical Secondary Standard; Certified Reference Material
|
|
| CAS | 108-10-1 | |
| PubChem CID | 7909 | |
| ChEMBL ID | CHEMBL285323 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 100.16 | ALogp: | 1.3 |
| HBD: | 0 | HBA: | 1 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 17.1 | Aromatic Rings: | 0 |
| Heavy Atoms: | 7 | QED Weighted: | 0.519 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.252 | MDCK Permeability: | 0.00003490 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.003 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.114 |
| 30% Bioavailability (F30%): | 0.018 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.995 | Plasma Protein Binding (PPB): | 31.57% |
| Volume Distribution (VD): | 0.953 | Fu: | 72.27% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.478 | CYP1A2-substrate: | 0.692 |
| CYP2C19-inhibitor: | 0.122 | CYP2C19-substrate: | 0.91 |
| CYP2C9-inhibitor: | 0.098 | CYP2C9-substrate: | 0.929 |
| CYP2D6-inhibitor: | 0.005 | CYP2D6-substrate: | 0.422 |
| CYP3A4-inhibitor: | 0.009 | CYP3A4-substrate: | 0.247 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 10.117 | Half-life (T1/2): | 0.799 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.014 | Human Hepatotoxicity (H-HT): | 0.119 |
| Drug-inuced Liver Injury (DILI): | 0.226 | AMES Toxicity: | 0.01 |
| Rat Oral Acute Toxicity: | 0.041 | Maximum Recommended Daily Dose: | 0.049 |
| Skin Sensitization: | 0.247 | Carcinogencity: | 0.072 |
| Eye Corrosion: | 0.97 | Eye Irritation: | 0.99 |
| Respiratory Toxicity: | 0.025 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
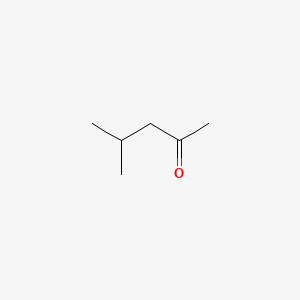
| ENC000376 | 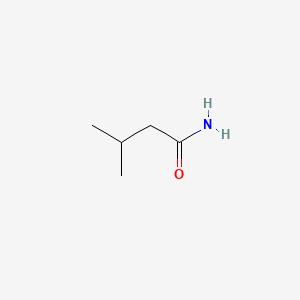 |
0.545 | D0ZK8H | 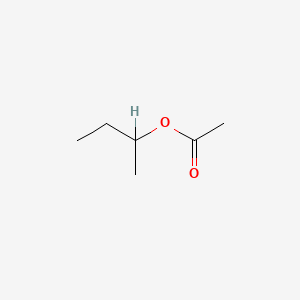 |
0.370 | ||
| ENC000351 | 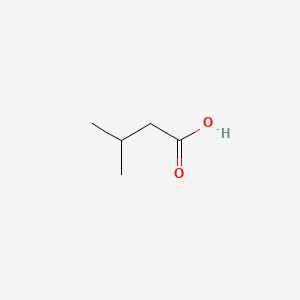 |
0.545 | D00WUF | 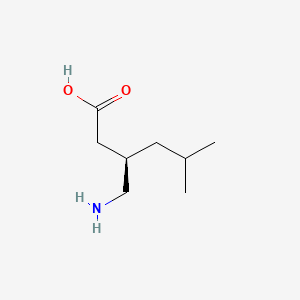 |
0.324 | ||
| ENC000225 | 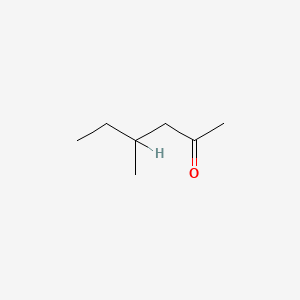 |
0.542 | D04MWJ | 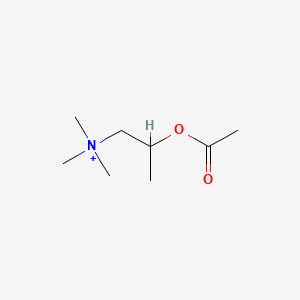 |
0.294 | ||
| ENC000685 | 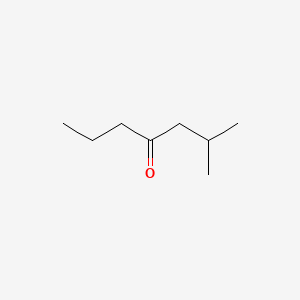 |
0.481 | D07ZTO | 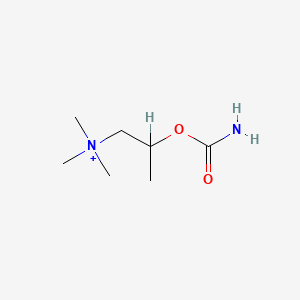 |
0.257 | ||
| ENC000241 | 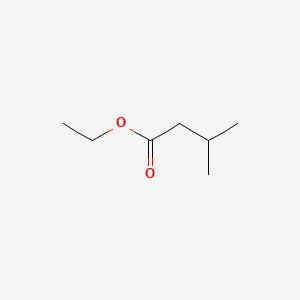 |
0.481 | D0G8SQ | 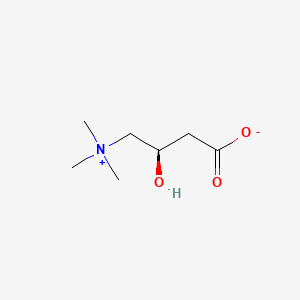 |
0.257 | ||
| ENC000246 | 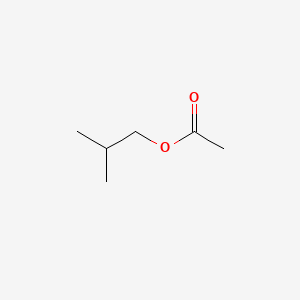 |
0.480 | D04CRL | 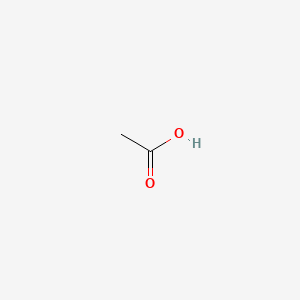 |
0.250 | ||
| ENC001735 | 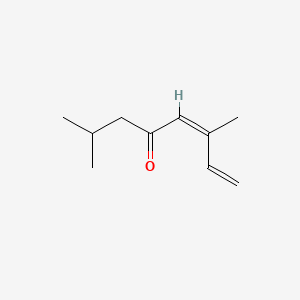 |
0.452 | D09PUL | 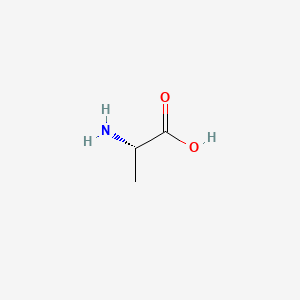 |
0.250 | ||
| ENC001734 | 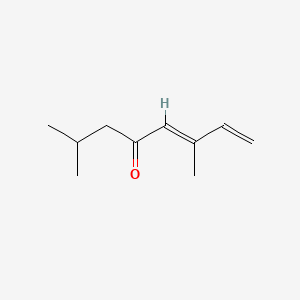 |
0.452 | D0XB8P | 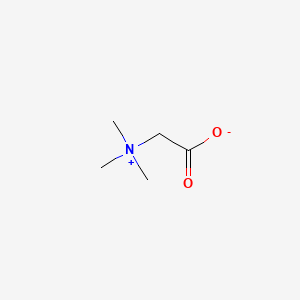 |
0.241 | ||
| ENC000603 | 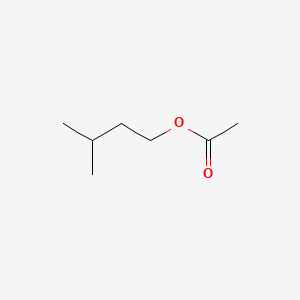 |
0.429 | D00ZOF | 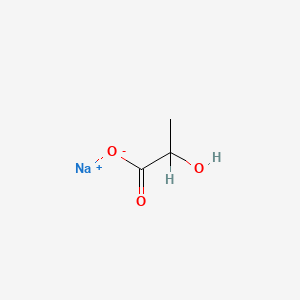 |
0.240 | ||
| ENC000397 | 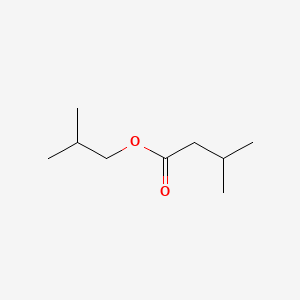 |
0.406 | D08QGD | 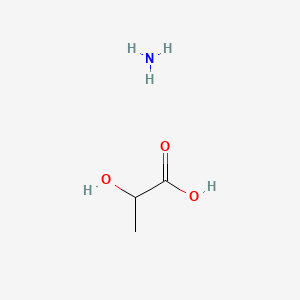 |
0.240 | ||