NPs Basic Information
|
Name |
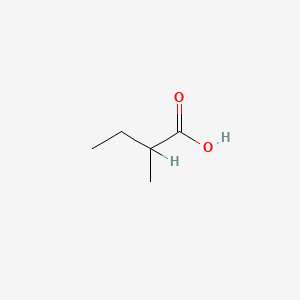
2-Methylbutanoic acid
|
| Molecular Formula | C5H10O2 | |
| IUPAC Name* |
2-methylbutanoic acid
|
|
| SMILES |
CCC(C)C(=O)O
|
|
| InChI |
InChI=1S/C5H10O2/c1-3-4(2)5(6)7/h4H,3H2,1-2H3,(H,6,7)
|
|
| InChIKey |
WLAMNBDJUVNPJU-UHFFFAOYSA-N
|
|
| Synonyms |
2-Methylbutanoic acid; 2-METHYLBUTYRIC ACID; 116-53-0; DL-2-Methylbutyric acid; Butanoic acid, 2-methyl-; Ethylmethylacetic acid; Methylbutyric acid; Methylethylacetic acid; 2-Methyl butyric acid; 2-Methybutyric acid; Active valeric acid; 600-07-7; Butyric acid, 2-methyl-; Carbomer 934; alpha-Methylbutyric acid; (+/-)-2-Methylbutyric acid; 2-methyl-butanoic acid; FEMA No. 2695; Butanoic acid, methyl-; alpha-methyl butyric Acid; NSC 7304; 2-methyl-butyric acid; PX7ZNN5GXK; .alpha.-Methylbutyric acid; 9007-16-3; CHEBI:37070; NSC-7304; DSSTox_CID_1621; DSSTox_RID_76241; DSSTox_GSID_21621; Valeric acid, active; Methylbutyricacid; 2-Methylbutyric acid (VAN); CAS-116-53-0; Carbopol 934; Carbopol 974P; 2-Methylbutyric acid (natrual); (+)-2-methylbutanoic acid; UNII-PX7ZNN5GXK; EINECS 204-145-2; EINECS 209-982-7; (1)-2-Methylbutyric acid; BRN 1098537; AI3-24202; MFCD09029093; Ethylmethylacetate; 2-Ethylpropionate; 2-Methyl Butyrate; 2-METHYLBUTANOIC ACID (DL); Methyl butyric acid; 2-Methylbutanoicacid; MFCD00002669; DL-2-Methylbutyrate; 2-Ethylpropionic acid; D-2-Methyl Butyrate; D-2-Methylbutyricacid; DL-2-Methy Butyrate; DL-2-Methylbutyricacid; ethyl methyl acetic acid; butane-2-carboxylic acid; rac-2-methylbutanoic acid; D-2-Methyl Butyric acid; DL-2-Methy Butyric acid; 2-METHYLBUTYRICACID; (+/-)-2-Methylbutyrate; EC 204-145-2; SCHEMBL49960; 2-Methyl-Butyric Acid Anion; 2-Methylbutyric acid, 98%; Nat. 2-Methyl Butyric Acid; (RS)-2-methyl-butyric acid; 4-02-00-00889 (Beilstein Handbook Reference); MLS001055480; Carbomer 934 [USAN:NF]; CHEMBL1160012; DTXSID5021621; NSC7304; (.+/-.)-2-Methylbutanoic acid; HMS2270O06; 2-METHYLBUTYRIC ACID, DL-; 2-METHYLBUTYRIC ACID [FCC]; 2-METHYLBUTYRIC ACID [FHFI]; NATURAL 2-METHYLBUTYRIC ACID; Tox21_201807; Tox21_303584; LMFA01020072; 2-Methylbutyric acid, >=98%, FG; Butanoic acid, 2-methyl-, (+ -); DL-.ALPHA.-METHYLBUTYRIC ACID; AKOS000121120; AKOS016843247; CS-W001942; SB47880; NCGC00090971-01; NCGC00090971-02; NCGC00257513-01; NCGC00259356-01; 2-Methylbutyric acid, analytical standard; AM802977; SMR000112113; SY115833; DB-003300; FT-0604458; FT-0605255; FT-0608333; FT-0671578; FT-0671579; M0181; EN300-27063; C18319; Q209433; (+/-)-2-Methylbutyric acid natural, >=98%, FG; J-509893; (+/-)-2-Methylbutyric acid, natural, >=98%, FG; F0001-0289; Z237374874
|
|
| CAS | 116-53-0 | |
| PubChem CID | 8314 | |
| ChEMBL ID | CHEMBL1160012 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 102.13 | ALogp: | 1.2 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 37.3 | Aromatic Rings: | 0 |
| Heavy Atoms: | 7 | QED Weighted: | 0.574 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.65 | MDCK Permeability: | 0.00009950 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.007 |
| Human Intestinal Absorption (HIA): | 0.007 | 20% Bioavailability (F20%): | 0.001 |
| 30% Bioavailability (F30%): | 0.003 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.971 | Plasma Protein Binding (PPB): | 43.15% |
| Volume Distribution (VD): | 0.37 | Fu: | 52.39% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.026 | CYP1A2-substrate: | 0.33 |
| CYP2C19-inhibitor: | 0.028 | CYP2C19-substrate: | 0.278 |
| CYP2C9-inhibitor: | 0.006 | CYP2C9-substrate: | 0.814 |
| CYP2D6-inhibitor: | 0.013 | CYP2D6-substrate: | 0.19 |
| CYP3A4-inhibitor: | 0.01 | CYP3A4-substrate: | 0.069 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 6.575 | Half-life (T1/2): | 0.81 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.008 | Human Hepatotoxicity (H-HT): | 0.101 |
| Drug-inuced Liver Injury (DILI): | 0.056 | AMES Toxicity: | 0.006 |
| Rat Oral Acute Toxicity: | 0.199 | Maximum Recommended Daily Dose: | 0.01 |
| Skin Sensitization: | 0.179 | Carcinogencity: | 0.047 |
| Eye Corrosion: | 0.985 | Eye Irritation: | 0.994 |
| Respiratory Toxicity: | 0.199 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000001 | 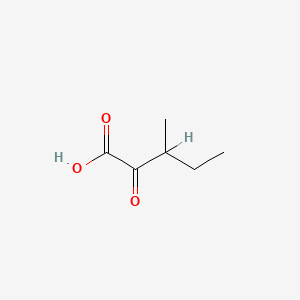 |
0.560 | D09PUL | 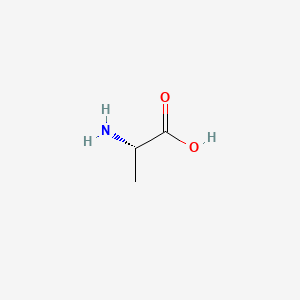 |
0.429 | ||
| ENC000149 | 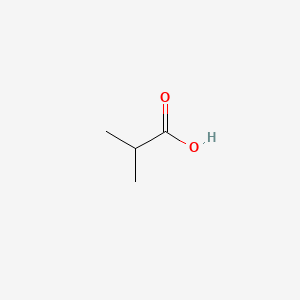 |
0.500 | D08QGD | 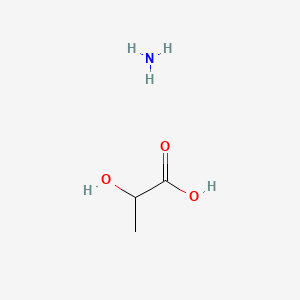 |
0.409 | ||
| ENC000141 | 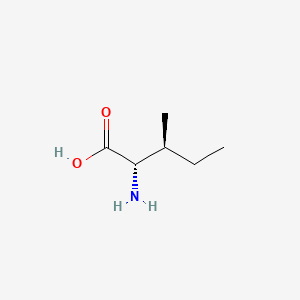 |
0.500 | D0ZK8H | 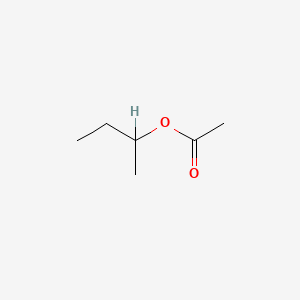 |
0.370 | ||
| ENC000771 | 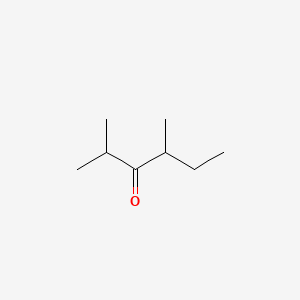 |
0.444 | D0Y3KG | 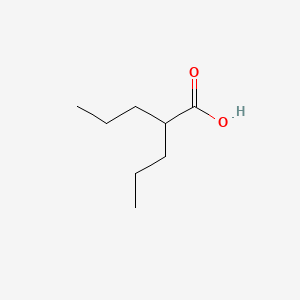 |
0.344 | ||
| ENC000037 | 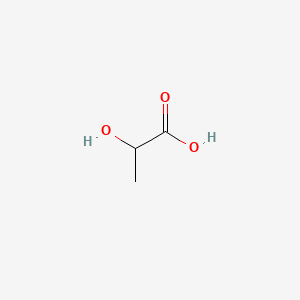 |
0.429 | D04CRL | 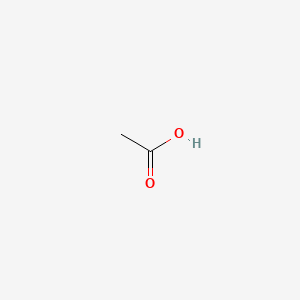 |
0.316 | ||
| ENC000351 | 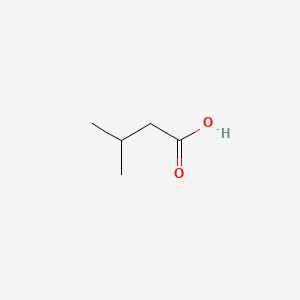 |
0.417 | D08HZC | 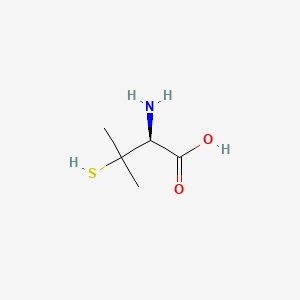 |
0.310 | ||
| ENC000780 | 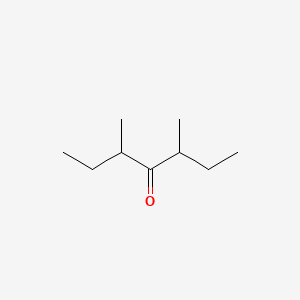 |
0.400 | D02UDJ | 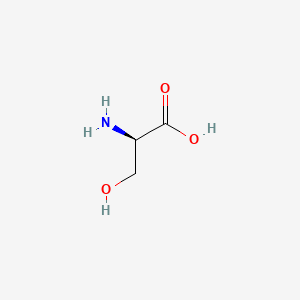 |
0.308 | ||
| ENC000058 | 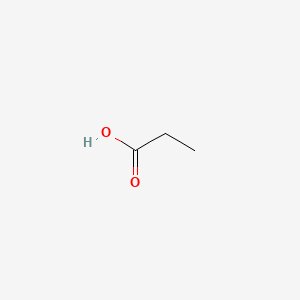 |
0.400 | D0P0QK | 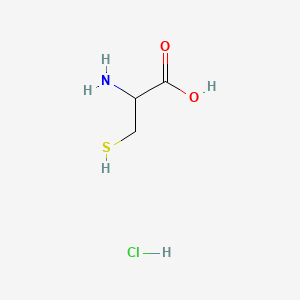 |
0.296 | ||
| ENC000306 | 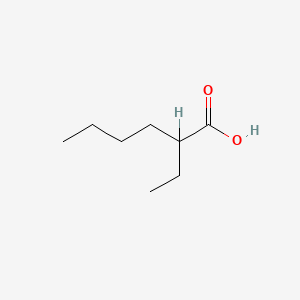 |
0.387 | D00ZOF | 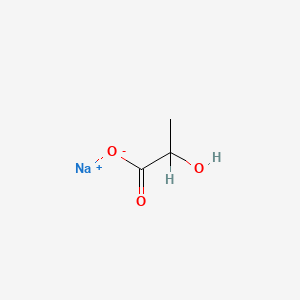 |
0.292 | ||
| ENC000824 | 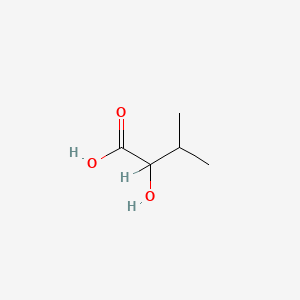 |
0.385 | D01OPV |  |
0.290 | ||