NPs Basic Information
|
Name |
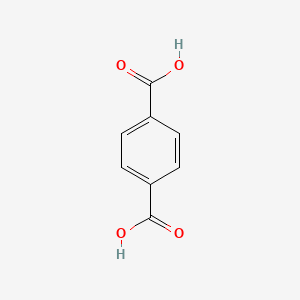
Terephthalic acid
|
| Molecular Formula | C8H6O4 | |
| IUPAC Name* |
terephthalic acid
|
|
| SMILES |
C1=CC(=CC=C1C(=O)O)C(=O)O
|
|
| InChI |
InChI=1S/C8H6O4/c9-7(10)5-1-2-6(4-3-5)8(11)12/h1-4H,(H,9,10)(H,11,12)
|
|
| InChIKey |
KKEYFWRCBNTPAC-UHFFFAOYSA-N
|
|
| Synonyms |
TEREPHTHALIC ACID; 100-21-0; p-Phthalic acid; 1,4-Benzenedicarboxylic acid; benzene-1,4-dicarboxylic acid; p-Dicarboxybenzene; p-Benzenedicarboxylic acid; p-Carboxybenzoic acid; Acide terephtalique; Tephthol; 1,4-dicarboxybenzene; Kyselina tereftalova; WR 16262; TA-33MP; 4-Carboxybenzoic Acid; NSC 36973; HSDB 834; p-Phthalate; TA 12; Kyselina terftalova; Benzene-p-dicarboxylic acid; 6S7NKZ40BQ; CHEBI:15702; NSC-36973; para-Phthalic acid; DSSTox_CID_6080; DSSTox_RID_78007; DSSTox_GSID_26080; Acide terephtalique [French]; Kyselina tereftalova [Czech]; CAS-100-21-0; CCRIS 2786; EINECS 202-830-0; UNII-6S7NKZ40BQ; BRN 1909333; terephtalic acid; AI3-16108; P-Phthelate; P-Phthelic acid; UB7; MFCD00002558; p-Benzenedicarboxylate; terephthalsäure; Benzene-p-dicarboxylate; benzene-1,4-dioic acid; WLN: QVR DVQ; Terephthalic acid, 97%; Terephthalic acid, 98%; EC 202-830-0; SCHEMBL1655; para-benzenedicarboxylic acid; Benzene, p-dicarboxylic acid; 4-09-00-03301 (Beilstein Handbook Reference); BIDD:ER0245; tere-Phthalic Acid (Sublimed); TEREPHTHALIC ACID [MI]; CHEMBL1374420; DTXSID6026080; TEREPHTHALIC ACID [HSDB]; Benzene, 1,4-Dicarboxylic acid; p-Dicarboxybenzene p-Phthalic acid; BCP06429; NSC36973; STR02759; Tox21_201659; Tox21_303229; s6251; STL281856; ZINC12358714; Terephthalic acid, analytical standard; AKOS000119464; CS-W010814; HY-W010098; NCGC00091618-01; NCGC00091618-02; NCGC00091618-03; NCGC00257014-01; NCGC00259208-01; AC-10250; BP-21157; FT-0674866; FT-0773240; T0166; EN300-18042; C06337; Terephthalic acid, SAJ special grade, >=98.0%; A852800; AE-562/40217759; Q408984; Terephthalic acid, Vetec(TM) reagent grade, 98%; Z57127536
|
|
| CAS | 100-21-0 | |
| PubChem CID | 7489 | |
| ChEMBL ID | CHEMBL1374420 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 166.13 | ALogp: | 2.0 |
| HBD: | 2 | HBA: | 4 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 74.6 | Aromatic Rings: | 1 |
| Heavy Atoms: | 12 | QED Weighted: | 0.698 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.694 | MDCK Permeability: | 0.00001390 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.046 | 20% Bioavailability (F20%): | 0.007 |
| 30% Bioavailability (F30%): | 0.259 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.367 | Plasma Protein Binding (PPB): | 43.48% |
| Volume Distribution (VD): | 0.217 | Fu: | 46.78% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.024 | CYP1A2-substrate: | 0.038 |
| CYP2C19-inhibitor: | 0.036 | CYP2C19-substrate: | 0.032 |
| CYP2C9-inhibitor: | 0.018 | CYP2C9-substrate: | 0.047 |
| CYP2D6-inhibitor: | 0.028 | CYP2D6-substrate: | 0.036 |
| CYP3A4-inhibitor: | 0.018 | CYP3A4-substrate: | 0.015 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 1.169 | Half-life (T1/2): | 0.934 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.065 | Human Hepatotoxicity (H-HT): | 0.343 |
| Drug-inuced Liver Injury (DILI): | 0.876 | AMES Toxicity: | 0.018 |
| Rat Oral Acute Toxicity: | 0.198 | Maximum Recommended Daily Dose: | 0.002 |
| Skin Sensitization: | 0.228 | Carcinogencity: | 0.013 |
| Eye Corrosion: | 0.012 | Eye Irritation: | 0.992 |
| Respiratory Toxicity: | 0.1 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000007 | 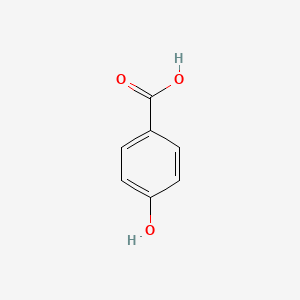 |
0.595 | D06OAV | 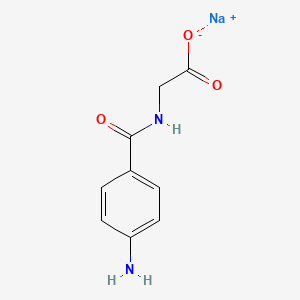 |
0.365 | ||
| ENC002802 | 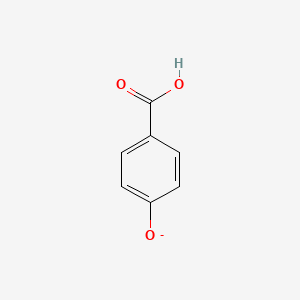 |
0.553 | D0L7FM | 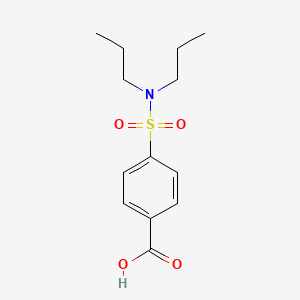 |
0.361 | ||
| ENC000468 | 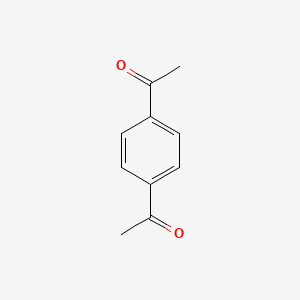 |
0.524 | D0NF1U | 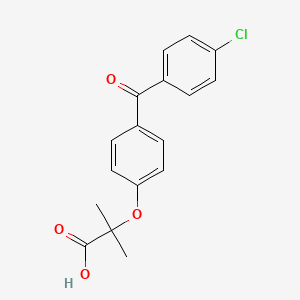 |
0.353 | ||
| ENC005264 | 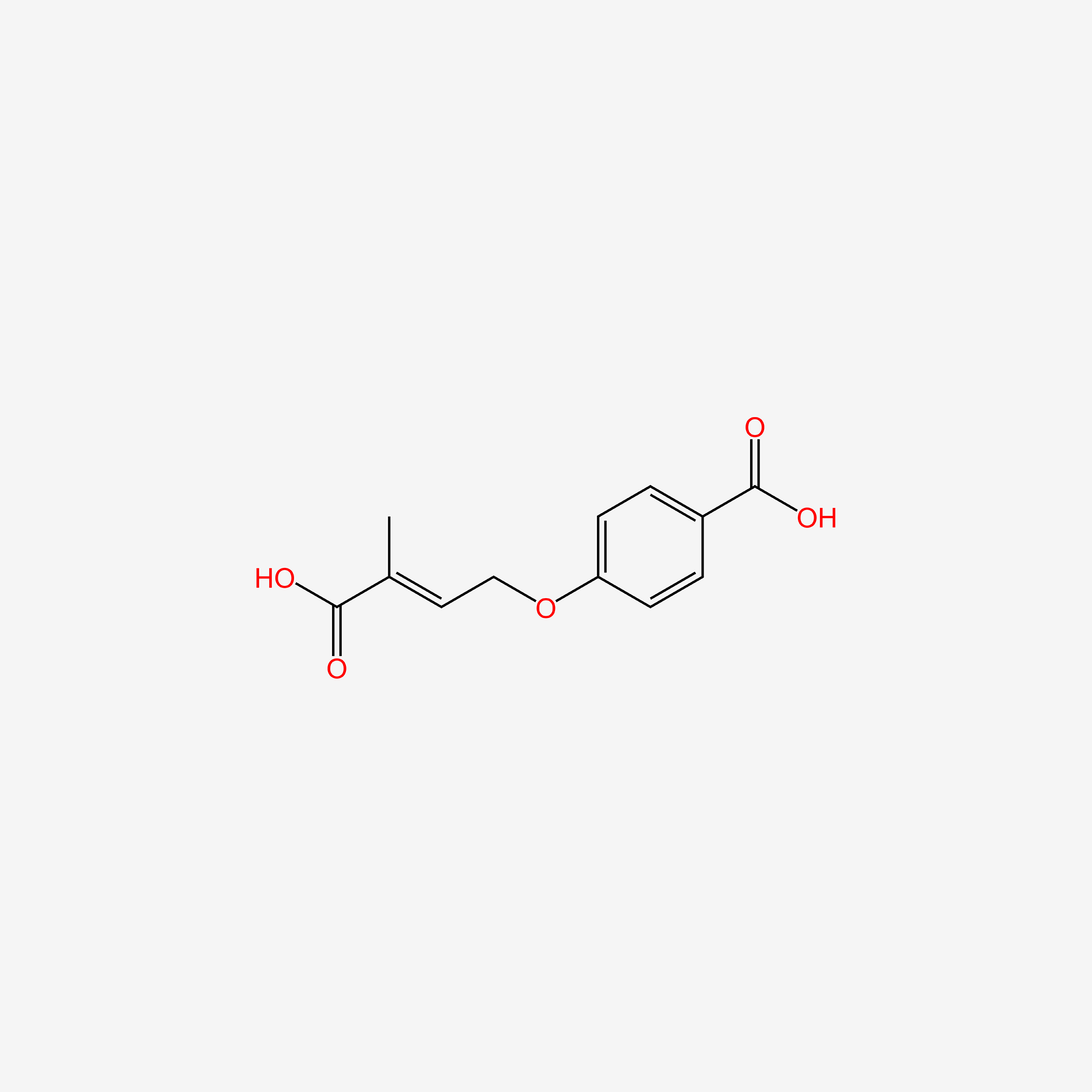 |
0.500 | D02AQY | 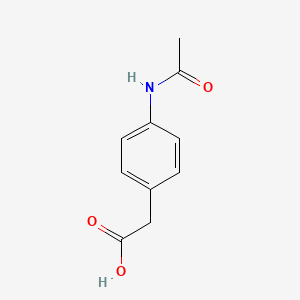 |
0.346 | ||
| ENC000055 | 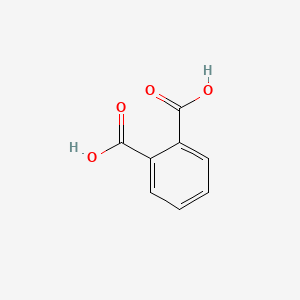 |
0.455 | D07BPS | 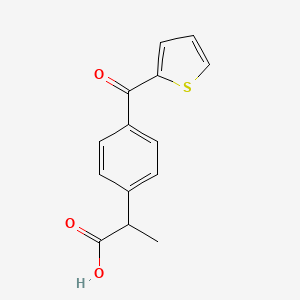 |
0.344 | ||
| ENC005265 | 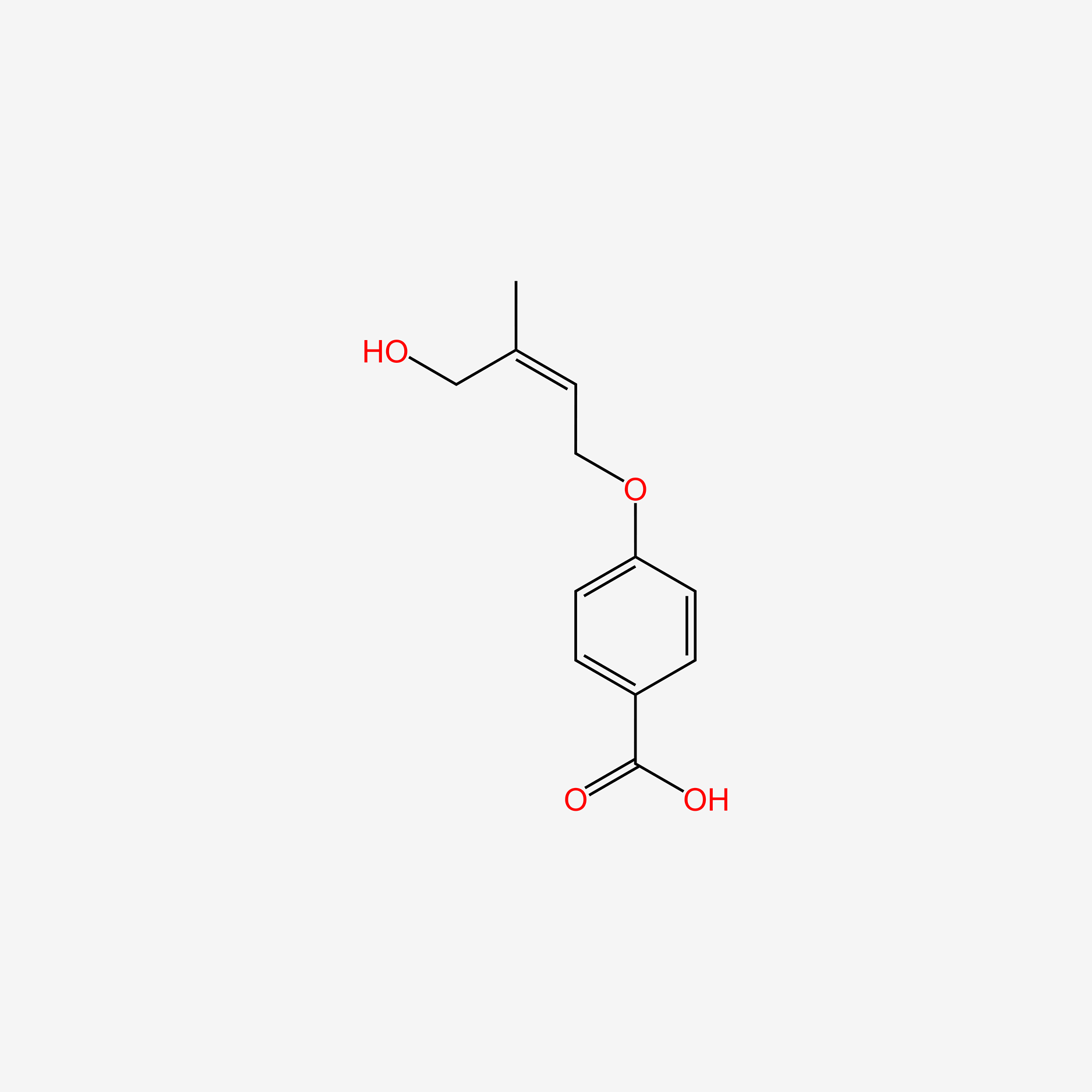 |
0.434 | D07HBX |  |
0.341 | ||
| ENC005266 | 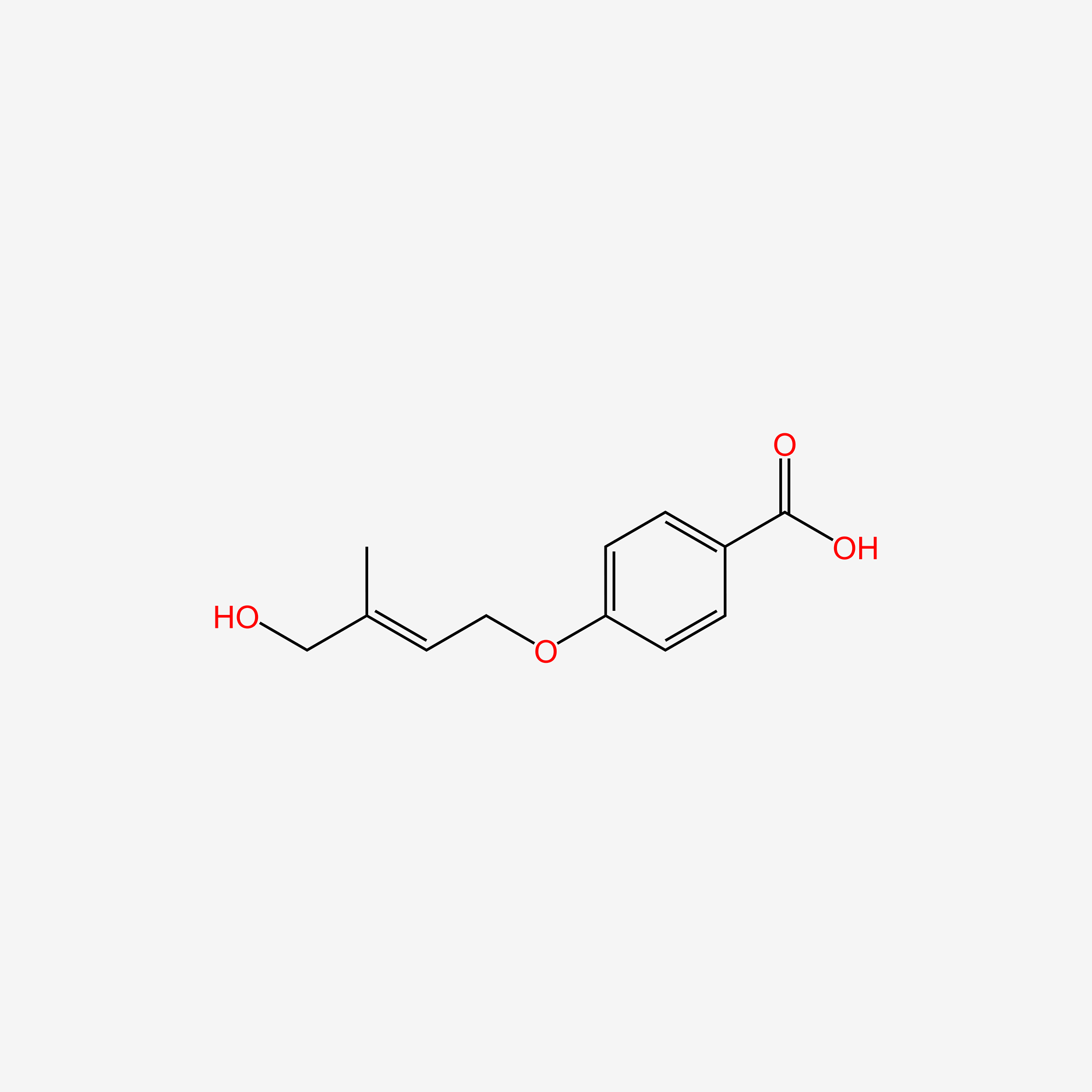 |
0.434 | D0GY5Z | 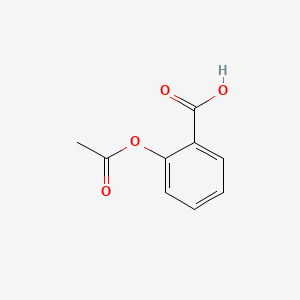 |
0.340 | ||
| ENC000348 | 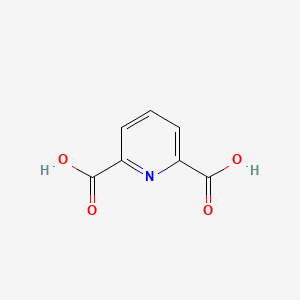 |
0.422 | D09BHB | 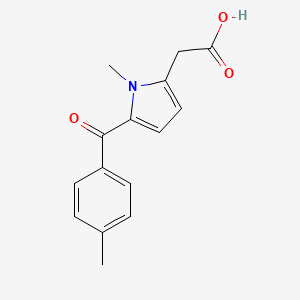 |
0.333 | ||
| ENC002433 | 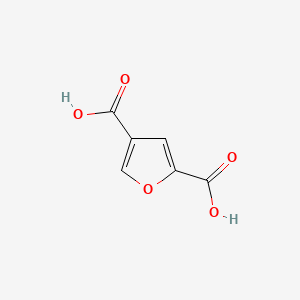 |
0.419 | D0Q8ZX | 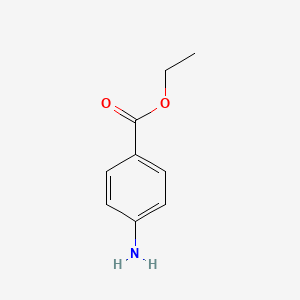 |
0.327 | ||
| ENC003949 |  |
0.407 | D06NVJ | 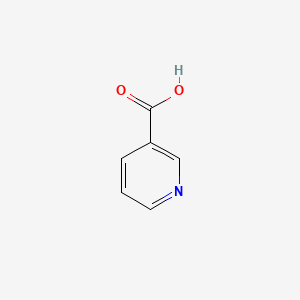 |
0.326 | ||