NPs Basic Information
|
Name |
2-Methoxy-4-methylphenol
|
| Molecular Formula | C8H10O2 | |
| IUPAC Name* |
2-methoxy-4-methylphenol
|
|
| SMILES |
CC1=CC(=C(C=C1)O)OC
|
|
| InChI |
InChI=1S/C8H10O2/c1-6-3-4-7(9)8(5-6)10-2/h3-5,9H,1-2H3
|
|
| InChIKey |
PETRWTHZSKVLRE-UHFFFAOYSA-N
|
|
| Synonyms |
2-METHOXY-4-METHYLPHENOL; 93-51-6; Creosol; 4-Methylguaiacol; 2-Methoxy-p-cresol; 4-Methyl guaiacol; Phenol, 2-methoxy-4-methyl-; Homoguaiacol; p-Methylguaiacol; p-Creosol; 2-Methoxy-4-cresol; 4-Hydroxy-3-methoxytoluene; p-Cresol, 2-methoxy-; 4-Methyl-2-methoxyphenol; 2-methoxy-4-methyl-phenol; 3-Methoxy-4-hydroxytoluene; Rohkcrsol; Kreosol; Cresolum drudum; 4-Hydroxy-3-methoxy-1-methylbenzene; Valspice; Homocatechol monomethyl ether; 1-Hydroxy-2-methoxy-4-methylbenzene; FEMA No. 2671; 2-Hydroxy-5-methylanisole; NSC 4969; MFCD00002378; W9GW1KZG6N; 2-methoxy-4-methyl phenol; 2-2-Methoxy-4-methylphenol; Phenol, 4-methyl-2-methoxy; CHEBI:89886; NSC-4969; Kreosol [German]; EINECS 202-252-9; UNII-W9GW1KZG6N; BRN 1862340; P-Methylguaicol; AI3-15891; Homocatechol methyl ester; CREOSOL [MI]; 3-Methoxy-4-methyl-Phenol; DSSTox_CID_27105; DSSTox_RID_82115; DSSTox_GSID_47105; SCHEMBL92236; 2-METHOXY-P- CRESOL; CHEMBL3182715; DTXSID6047105; 2-Methoxy-4-methylphenol, 9CI; FEMA 2671; NSC4969; BCP19090; ZINC3875629; 2-Methoxy-4-methylphenol (creosol); Tox21_302681; 2-Methoxy-4-methylphenol, >=98%; AKOS000120520; CS-W021711; HY-W040971; CAS-93-51-6; 2-METHOXY-4-METHYLPHENOL [FHFI]; NCGC00256731-01; 2-Methoxy-4-methylphenol, >=98%, FG; AS-15967; SY036778; 2-Methoxy-4-methylphenol (4-methylguaiacol); 4-Methyl-2-methoxyphenol (4-methylguaiacol); FT-0612795; M0114; 2-Methoxy-4-methylphenol, analytical standard; EN300-18155; 2-Methoxy-4-methylphenol, natural, 97%, FG; D70655; Q403037; Q-100894; F0001-2237
|
|
| CAS | 93-51-6 | |
| PubChem CID | 7144 | |
| ChEMBL ID | CHEMBL3182715 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 138.16 | ALogp: | 1.3 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 29.5 | Aromatic Rings: | 1 |
| Heavy Atoms: | 10 | QED Weighted: | 0.645 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.358 | MDCK Permeability: | 0.00002420 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.741 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.025 |
| 30% Bioavailability (F30%): | 0.035 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.512 | Plasma Protein Binding (PPB): | 83.78% |
| Volume Distribution (VD): | 1.007 | Fu: | 11.43% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.913 | CYP1A2-substrate: | 0.95 |
| CYP2C19-inhibitor: | 0.513 | CYP2C19-substrate: | 0.837 |
| CYP2C9-inhibitor: | 0.134 | CYP2C9-substrate: | 0.863 |
| CYP2D6-inhibitor: | 0.428 | CYP2D6-substrate: | 0.913 |
| CYP3A4-inhibitor: | 0.063 | CYP3A4-substrate: | 0.421 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 13.906 | Half-life (T1/2): | 0.885 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.029 | Human Hepatotoxicity (H-HT): | 0.056 |
| Drug-inuced Liver Injury (DILI): | 0.104 | AMES Toxicity: | 0.049 |
| Rat Oral Acute Toxicity: | 0.259 | Maximum Recommended Daily Dose: | 0.091 |
| Skin Sensitization: | 0.731 | Carcinogencity: | 0.655 |
| Eye Corrosion: | 0.968 | Eye Irritation: | 0.992 |
| Respiratory Toxicity: | 0.465 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
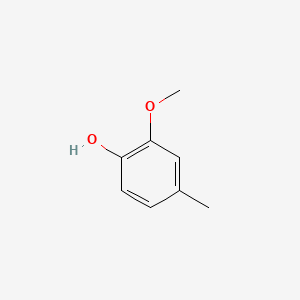
| ENC000027 | 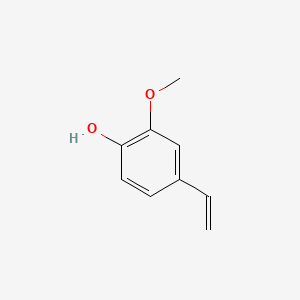 |
0.583 | D06GIP | 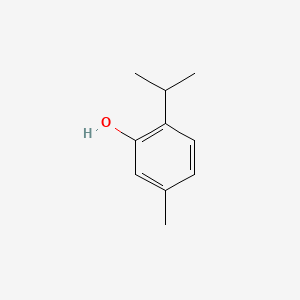 |
0.474 | ||
| ENC000068 |  |
0.583 | D0E9CD |  |
0.462 | ||
| ENC001056 | 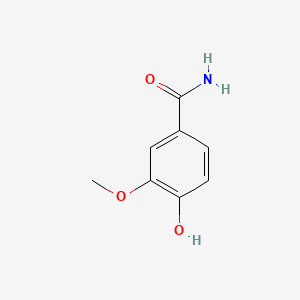 |
0.553 | D09GYT | 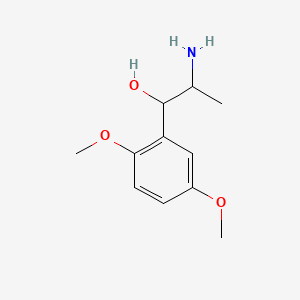 |
0.340 | ||
| ENC000296 | 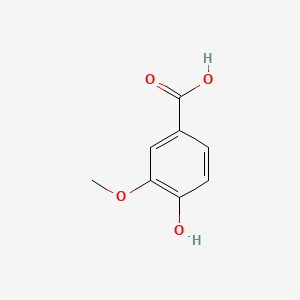 |
0.553 | D05VIX | 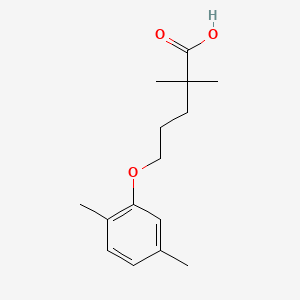 |
0.339 | ||
| ENC000471 | 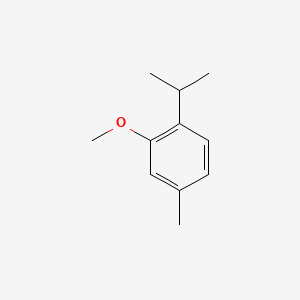 |
0.553 | D0U5CE | 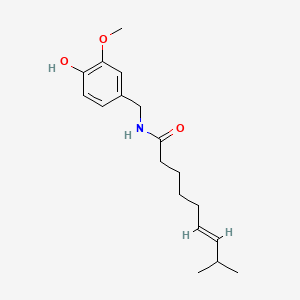 |
0.333 | ||
| ENC000329 | 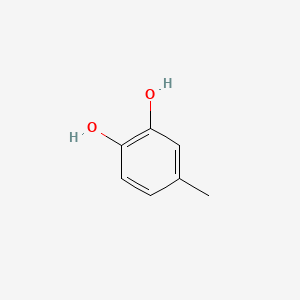 |
0.545 | D03LGG | 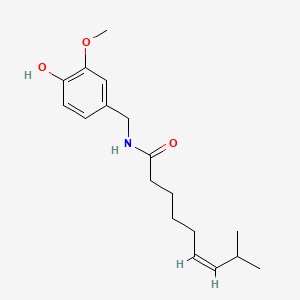 |
0.333 | ||
| ENC000734 | 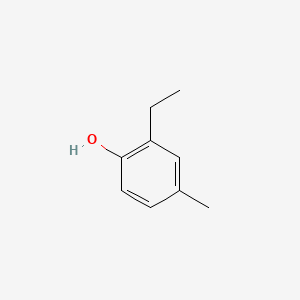 |
0.543 | D0U0OT | 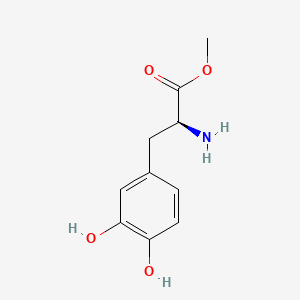 |
0.314 | ||
| ENC000095 | 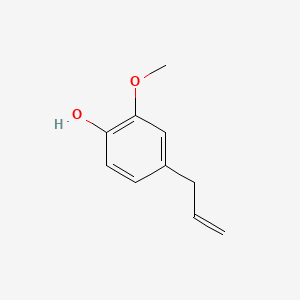 |
0.538 | D0C4YC | 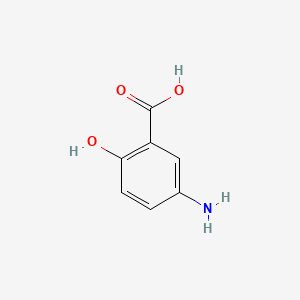 |
0.302 | ||
| ENC000507 | 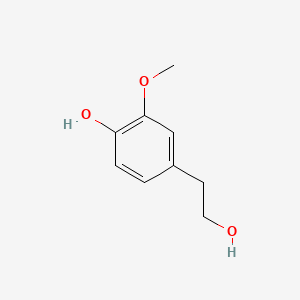 |
0.538 | D0T7OW | 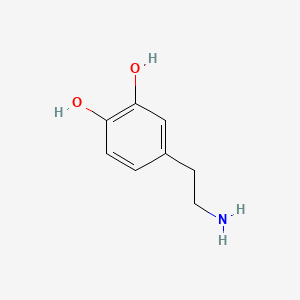 |
0.295 | ||
| ENC000777 | 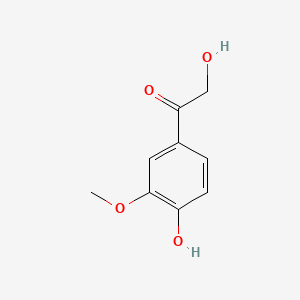 |
0.512 | D04PHC | 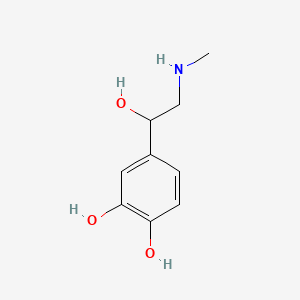 |
0.292 | ||