NPs Basic Information
|
Name |
Eugenol
|
| Molecular Formula | C10H12O2 | |
| IUPAC Name* |
2-methoxy-4-prop-2-enylphenol
|
|
| SMILES |
COC1=C(C=CC(=C1)CC=C)O
|
|
| InChI |
InChI=1S/C10H12O2/c1-3-4-8-5-6-9(11)10(7-8)12-2/h3,5-7,11H,1,4H2,2H3
|
|
| InChIKey |
RRAFCDWBNXTKKO-UHFFFAOYSA-N
|
|
| Synonyms |
eugenol; 97-53-0; 4-Allyl-2-methoxyphenol; 4-Allylguaiacol; Eugenic acid; Allylguaiacol; Caryophyllic acid; p-Allylguaiacol; 2-Methoxy-4-prop-2-enylphenol; p-Eugenol; Engenol; 2-Methoxy-4-allylphenol; Phenol, 2-methoxy-4-(2-propenyl)-; 2-Methoxy-4-(2-propenyl)phenol; 1,3,4-Eugenol; 4-Allylcatechol-2-methyl ether; 1-Hydroxy-2-methoxy-4-allylbenzene; 5-Allylguaiacol; Synthetic eugenol; 2-Methoxy-1-hydroxy-4-allylbenzene; 4-Allyl-1-hydroxy-2-methoxybenzene; 2-methoxy-4-(prop-2-en-1-yl)phenol; 1-Hydroxy-2-methoxy-4-prop-2-enylbenzene; Eugenol (natural); FEMA No. 2467; 4-Hydroxy-3-methoxy-1-allylbenzene; 2-Hydroxy-5-allylanisole; bioxeda; 4-Allylcatechol 2-methyl ether; 4-Hydroxy-3-methoxyallylbenzene; 2-Methoxy-4-(2-propen-1-yl)phenol; Phenol, 4-allyl-2-methoxy-; NCI-C50453; 1-allyl-4-hydroxy-3-methoxybenzene; 1-Allyl-3-methoxy-4-hydroxybenzene; 2-Metoksy-4-allilofenol; Caryophillic acid; FA 100; Eugenol [USP]; NSC 209525; CHEBI:4917; Phenol, 2-methoxy-4-(2-propen-1-yl)-; NSC-8895; NSC-209525; CHEMBL42710; 2-Methoxy-4-(3-propenyl)phenol; 3T8H1794QW; Eugenol (USP); NCGC00091449-05; DSSTox_CID_617; DSSTox_RID_75693; DSSTox_GSID_20617; Eugenol [USAN]; WLN: 1U2R DQ CO1; Caswell No. 456BC; FEMA Number 2467; CAS-97-53-0; 38219-15-7; CCRIS 306; HSDB 210; 2-Metoksy-4-allilofenol [Polish]; SR-05000002043; EINECS 202-589-1; MFCD00008654; EPA Pesticide Chemical Code 102701; BRN 1366759; UNII-3T8H1794QW; AI3-00086; Eugenol,(S); 4-allyl-2methoxyphenol; 3s0e; EUGENOL [VANDF]; EUGENOL [FHFI]; EUGENOL [HSDB]; EUGENOL [IARC]; EUGENOL [INCI]; EUGENOL [FCC]; EUGENOL [II]; EUGENOL [MI]; EUGENOL [MART.]; Spectrum2_001264; Spectrum3_000646; Spectrum4_001783; Spectrum5_000425; EUGENOL [USP-RS]; EUGENOL [WHO-DD]; 4-allyl-2-methoxy-Phenol; bmse010053; Epitope ID:114091; Eugenol, puriss., 98%; EC 202-589-1; SCHEMBL20361; BSPBio_002251; KBioGR_002327; MLS000028901; BIDD:ER0696; DivK1c_000692; SPECTRUM1500296; SPBio_001228; EUGENOL [EP MONOGRAPH]; GTPL2425; ZINC1411; EUGENOL [USP MONOGRAPH]; DTXSID9020617; HMS502C14; KBio1_000692; KBio3_001471; Eugenol, ReagentPlus(R), 99%; NSC8895; 4-(2-Propenyl)-2-methoxyphenol; Eugenol, natural, >=98%, FG; NINDS_000692; Eugenol, >=98%, FCC, FG; HMS1920O08; HMS2091F09; Pharmakon1600-01500296; HY-N0337; Tox21_111134; Tox21_202040; Tox21_300105; BBL027721; BDBM50164168; CCG-38827; NSC209525; NSC757030; s4706; STL371304; Eugenol, tested according to Ph.Eur.; 3-(3-methoxy-4-hydroxyphenyl)propene; AKOS000121354; Tox21_111134_1; CS-7807; DB09086; FS-2702; NSC-757030; SDCCGMLS-0066578.P001; IDI1_000692; Eugenol 1000 microg/mL in Acetonitrile; NCGC00091449-01; NCGC00091449-02; NCGC00091449-03; NCGC00091449-04; NCGC00091449-06; NCGC00091449-07; NCGC00091449-08; NCGC00091449-10; NCGC00253915-01; NCGC00259589-01; AC-34149; Eugenol, Vetec(TM) reagent grade, 98%; SMR000059114; SBI-0051381.P003; Eugenol, PESTANAL(R), analytical standard; A0232; FT-0615974; EN300-16622; D04117; AB00051992_02; A845719; Eugenol, primary pharmaceutical reference standard; Q423357; Eugenol, certified reference material, TraceCERT(R); Q-201105; SR-05000002043-1; SR-05000002043-2; BRD-K32977963-001-01-9; BRD-K32977963-001-03-5; Z56347226; EUGENOL (CONSTITUENT OF HOLY BASIL LEAF) [DSC]; Eugenol, European Pharmacopoeia (EP) Reference Standard; F0001-2306; 2-methoxy-4-(prop-2-en-1-yl)phenol4-allyl-2-methoxyphenol; EUGENOL (CONSTITUENT OF CINNAMOMUM CASSIA BARK) [DSC]; EUGENOL (CONSTITUENT OF CINNAMOMUM VERUM BARK) [DSC]; Eugenol, United States Pharmacopeia (USP) Reference Standard; Eugenol, Pharmaceutical Secondary Standard; Certified Reference Material
|
|
| CAS | 97-53-0 | |
| PubChem CID | 3314 | |
| ChEMBL ID | CHEMBL42710 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 164.2 | ALogp: | 2.0 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 3 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 29.5 | Aromatic Rings: | 1 |
| Heavy Atoms: | 12 | QED Weighted: | 0.696 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.373 | MDCK Permeability: | 0.00003010 |
| Pgp-inhibitor: | 0.003 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.007 | 20% Bioavailability (F20%): | 0.732 |
| 30% Bioavailability (F30%): | 0.967 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.188 | Plasma Protein Binding (PPB): | 92.12% |
| Volume Distribution (VD): | 0.833 | Fu: | 3.22% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.901 | CYP1A2-substrate: | 0.941 |
| CYP2C19-inhibitor: | 0.716 | CYP2C19-substrate: | 0.659 |
| CYP2C9-inhibitor: | 0.313 | CYP2C9-substrate: | 0.875 |
| CYP2D6-inhibitor: | 0.85 | CYP2D6-substrate: | 0.921 |
| CYP3A4-inhibitor: | 0.288 | CYP3A4-substrate: | 0.371 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 14.042 | Half-life (T1/2): | 0.887 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.017 | Human Hepatotoxicity (H-HT): | 0.036 |
| Drug-inuced Liver Injury (DILI): | 0.046 | AMES Toxicity: | 0.066 |
| Rat Oral Acute Toxicity: | 0.121 | Maximum Recommended Daily Dose: | 0.153 |
| Skin Sensitization: | 0.792 | Carcinogencity: | 0.814 |
| Eye Corrosion: | 0.713 | Eye Irritation: | 0.982 |
| Respiratory Toxicity: | 0.51 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
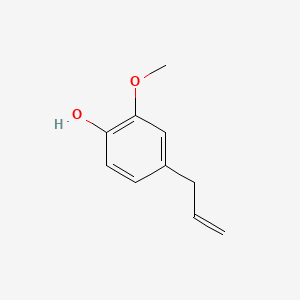
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000027 | 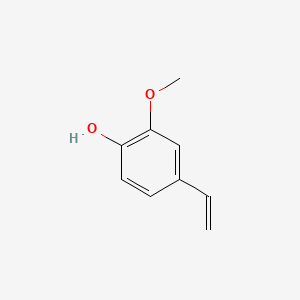 |
0.615 | D0E9CD |  |
0.432 | ||
| ENC000507 | 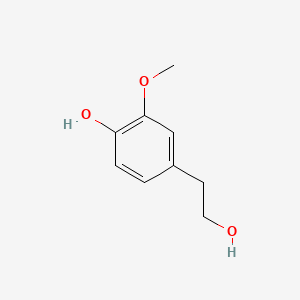 |
0.610 | D03LGG | 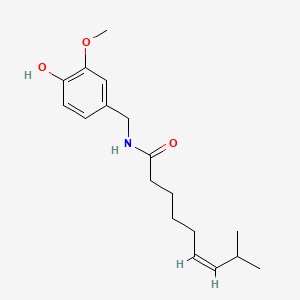 |
0.382 | ||
| ENC001052 | 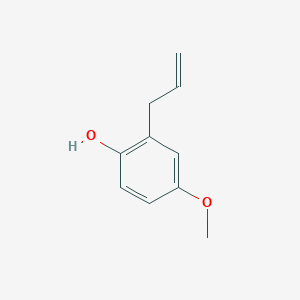 |
0.571 | D0U5CE | 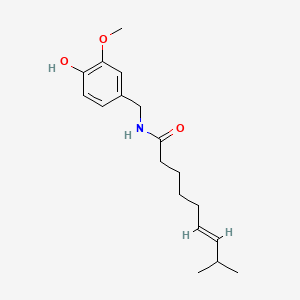 |
0.382 | ||
| ENC000172 | 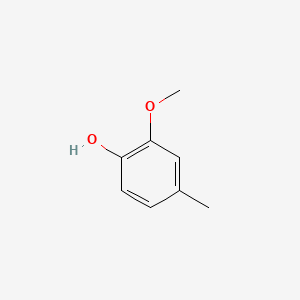 |
0.538 | D0U0OT | 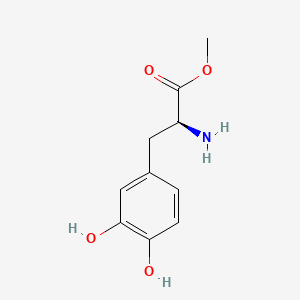 |
0.352 | ||
| ENC000068 | 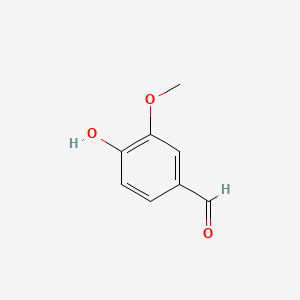 |
0.537 | D0C6OQ | 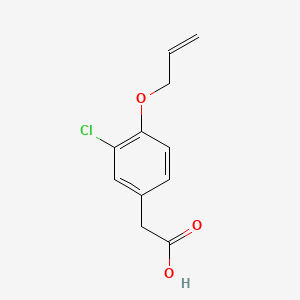 |
0.345 | ||
| ENC000325 | 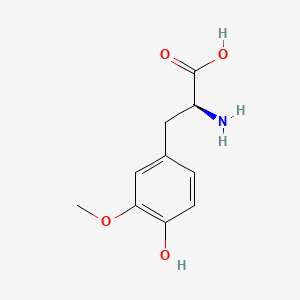 |
0.521 | D0T7OW | 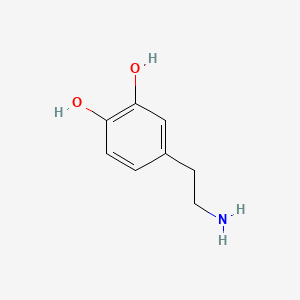 |
0.340 | ||
| ENC000310 | 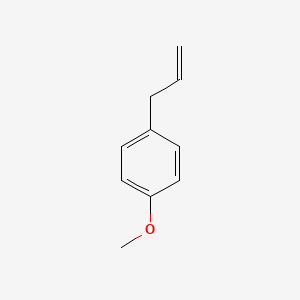 |
0.488 | D0BA6T | 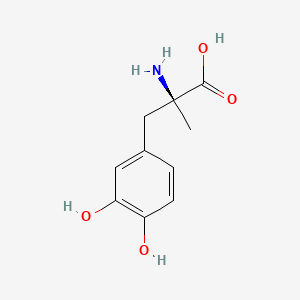 |
0.309 | ||
| ENC000777 | 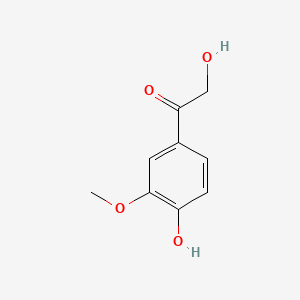 |
0.478 | D08HVR | 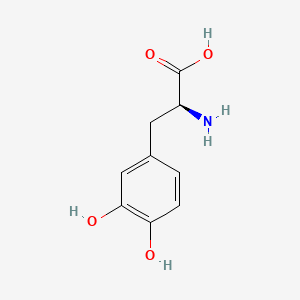 |
0.296 | ||
| ENC000296 | 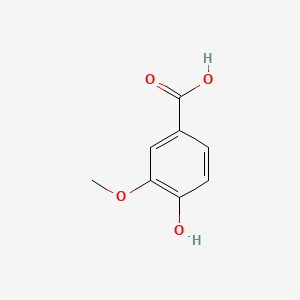 |
0.477 | D0Y6KO | 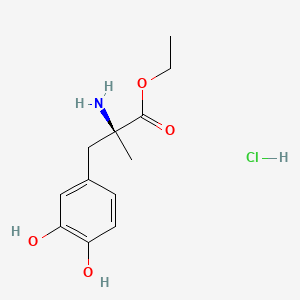 |
0.295 | ||
| ENC001056 | 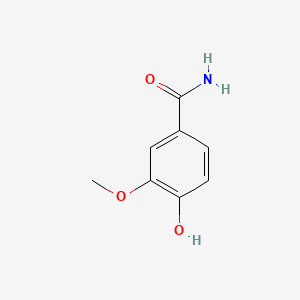 |
0.477 | D0P7JZ | 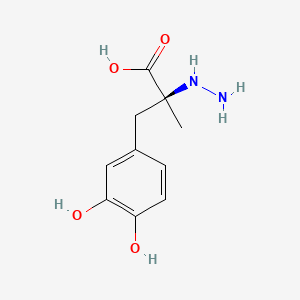 |
0.293 | ||