NPs Basic Information
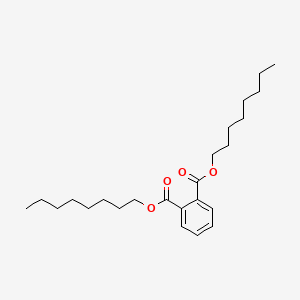
|
Name |
Dioctyl phthalate
|
| Molecular Formula | C24H38O4 | |
| IUPAC Name* |
dioctyl benzene-1,2-dicarboxylate
|
|
| SMILES |
CCCCCCCCOC(=O)C1=CC=CC=C1C(=O)OCCCCCCCC
|
|
| InChI |
InChI=1S/C24H38O4/c1-3-5-7-9-11-15-19-27-23(25)21-17-13-14-18-22(21)24(26)28-20-16-12-10-8-6-4-2/h13-14,17-18H,3-12,15-16,19-20H2,1-2H3
|
|
| InChIKey |
MQIUGAXCHLFZKX-UHFFFAOYSA-N
|
|
| Synonyms |
Dioctyl phthalate; DI-N-OCTYL PHTHALATE; 117-84-0; dioctyl benzene-1,2-dicarboxylate; DNOP; Vinicizer 85; Dinopol NOP; n-Octyl phthalate; Phthalic acid di-n-octyl ester; Phthalic acid, dioctyl ester; Dioctyl 1,2-benzenedicarboxylate; Dioctyl o-benzenedicarboxylate; Bis(n-octyl) phthalate; 1,2-Benzenedicarboxylic acid, 1,2-dioctyl ester; 1,2-Benzenedicarboxylic acid, dioctyl ester; RCRA waste number U107; Dioktylester kyseliny ftalove; NSC 15318; 1,2-Benzenedicarbonic acid, dioctyl ester; 8031-29-6; CHEBI:34679; 8X3RJ0527W; NSC-15318; NCGC00090781-02; DSSTox_CID_1956; DSSTox_RID_76425; DSSTox_GSID_21956; 68515-43-5; di-octyl phthalate; CAS-117-84-0; Di-n-octyl phthalate, analytical standard; CCRIS 6196; HSDB 1345; AI3-15071 (USDA); EINECS 204-214-7; Dioktylester kyseliny ftalove [Czech]; RCRA waste no. U107; BRN 1915994; Benzenedicarboxylic acid di-n-octyl ester; UNII-8X3RJ0527W; 1, dioctyl ester; Vinycizer 85; di-n-octylphthalate; Dioctyl o-phthalate; Phthalic acid dioctyl; SCHEMBL23053; BIDD:ER0319; CHEMBL1409747; DTXSID1021956; Phthalic acid, bis-n-octyl ester; NSC15318; ZINC8437697; DI-N-OCTYL PHTHALATE [HSDB]; Tox21_111020; Tox21_202233; Tox21_300549; Di-n-octyl phthalate, p.a., 99%; MFCD00015292; STL280370; AKOS015889916; 1,2-dioctyl benzene-1,2-dicarboxylate; NCGC00090781-01; NCGC00090781-03; NCGC00090781-04; NCGC00090781-05; NCGC00254360-01; NCGC00259782-01; Di-n-octyl phthalate, >=98.0% (GC); LS-15074; FT-0655747; FT-0667608; P0304; EN300-40135; 1,2-BENZENEDICARBOXYLIC ACID DIOCTYL ESTER; A803836; Q908490; J-003672; J-520376; F0001-0293; Z407875554; Di-n-octyl phthalate, certified reference material, TraceCERT(R)
|
|
| CAS | 117-84-0 | |
| PubChem CID | 8346 | |
| ChEMBL ID | CHEMBL1409747 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 390.6 | ALogp: | 9.1 |
| HBD: | 0 | HBA: | 4 |
| Rotatable Bonds: | 18 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 52.6 | Aromatic Rings: | 1 |
| Heavy Atoms: | 28 | QED Weighted: | 0.234 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.843 | MDCK Permeability: | 0.00001690 |
| Pgp-inhibitor: | 0.889 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.001 | 20% Bioavailability (F20%): | 1 |
| 30% Bioavailability (F30%): | 0.998 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.019 | Plasma Protein Binding (PPB): | 98.46% |
| Volume Distribution (VD): | 2.03 | Fu: | 1.33% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.213 | CYP1A2-substrate: | 0.183 |
| CYP2C19-inhibitor: | 0.579 | CYP2C19-substrate: | 0.051 |
| CYP2C9-inhibitor: | 0.151 | CYP2C9-substrate: | 0.816 |
| CYP2D6-inhibitor: | 0.491 | CYP2D6-substrate: | 0.048 |
| CYP3A4-inhibitor: | 0.418 | CYP3A4-substrate: | 0.053 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.99 | Half-life (T1/2): | 0.054 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.254 | Human Hepatotoxicity (H-HT): | 0.002 |
| Drug-inuced Liver Injury (DILI): | 0.231 | AMES Toxicity: | 0.004 |
| Rat Oral Acute Toxicity: | 0.002 | Maximum Recommended Daily Dose: | 0.005 |
| Skin Sensitization: | 0.948 | Carcinogencity: | 0.237 |
| Eye Corrosion: | 0.032 | Eye Irritation: | 0.987 |
| Respiratory Toxicity: | 0.053 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000156 | 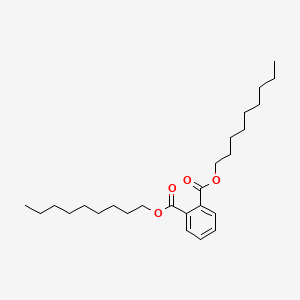 |
0.930 | D0K8CI | 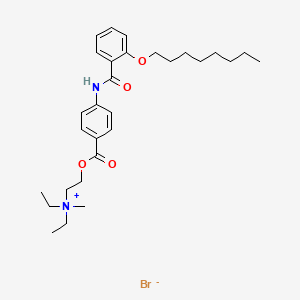 |
0.429 | ||
| ENC000164 | 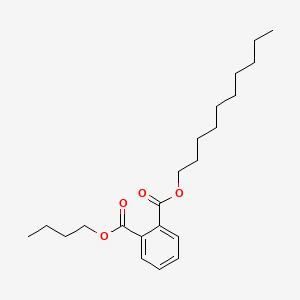 |
0.901 | D0Z5SM |  |
0.411 | ||
| ENC000669 | 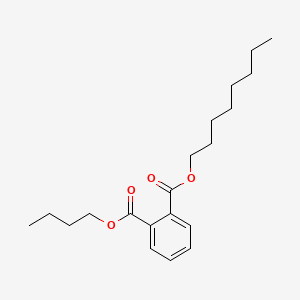 |
0.827 | D05ATI |  |
0.396 | ||
| ENC000293 | 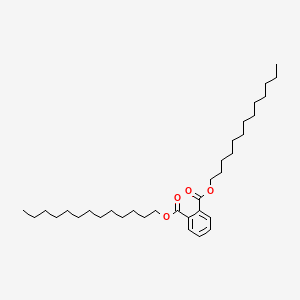 |
0.727 | D00MLW | 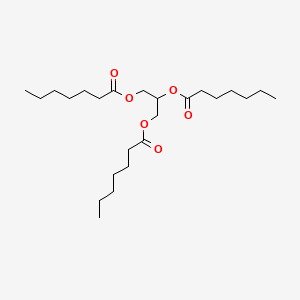 |
0.390 | ||
| ENC000090 | 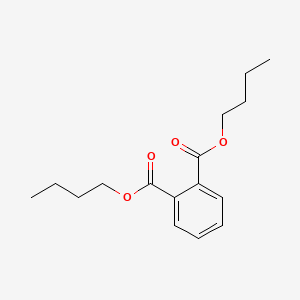 |
0.659 | D0P1RL | 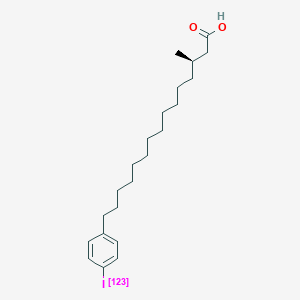 |
0.373 | ||
| ENC001801 | 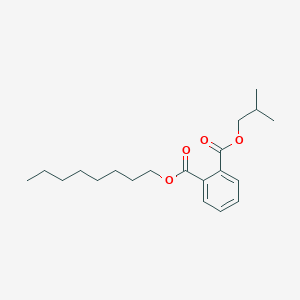 |
0.652 | D07ILQ |  |
0.373 | ||
| ENC000616 | 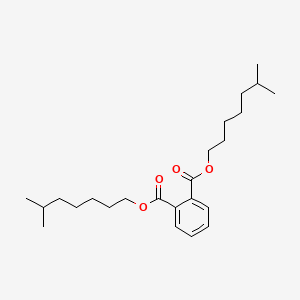 |
0.612 | D00FGR | 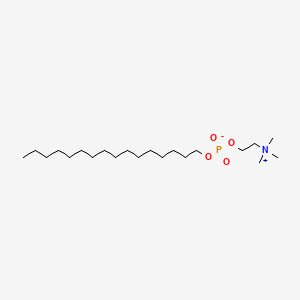 |
0.372 | ||
| ENC000158 | 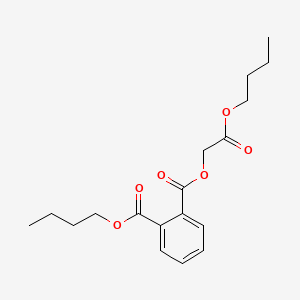 |
0.581 | D0OR6A | 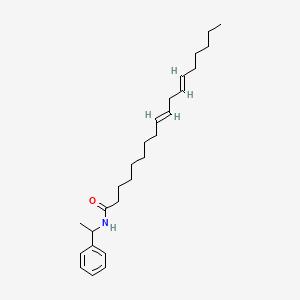 |
0.353 | ||
| ENC000601 | 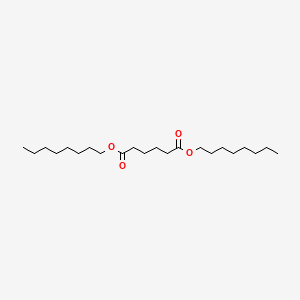 |
0.571 | D06ORU | 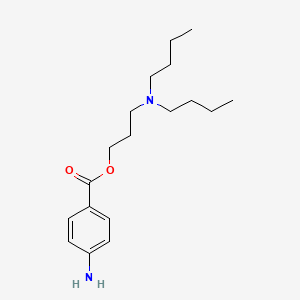 |
0.352 | ||
| ENC000157 | 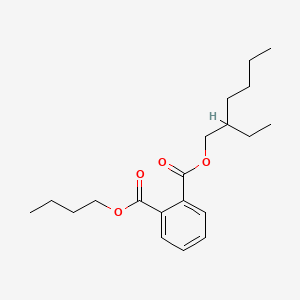 |
0.564 | D0O1PH |  |
0.352 | ||