NPs Basic Information
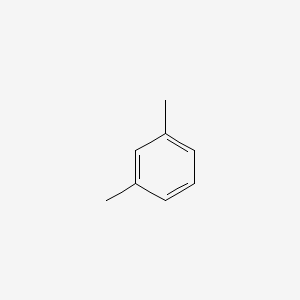
|
Name |
m-Xylene
|
| Molecular Formula | C8H10 | |
| IUPAC Name* |
1,3-xylene
|
|
| SMILES |
CC1=CC(=CC=C1)C
|
|
| InChI |
InChI=1S/C8H10/c1-7-4-3-5-8(2)6-7/h3-6H,1-2H3
|
|
| InChIKey |
IVSZLXZYQVIEFR-UHFFFAOYSA-N
|
|
| Synonyms |
M-XYLENE; 1,3-Dimethylbenzene; 108-38-3; 1,3-Xylene; meta-Xylene; m-Xylol; m-Dimethylbenzene; m-Methyltoluene; Benzene, 1,3-dimethyl-; 3-Xylene; 1,3-Dimethylbenzol; Santosol 150; m-Xylenes; 2,4-Xylene; NSC 61769; O9XS864HTE; CHEMBL286727; CHEBI:28488; NSC-61769; Xylol, Dimethylbenzene; DSSTox_CID_1446; DSSTox_RID_78091; DSSTox_GSID_26298; Xylene, m-; Naphtha Solvent from Coal Tar; Benzene, m-dimethyl-; CAS-108-38-3; CCRIS 907; HSDB 135; EINECS 203-576-3; EINECS 272-684-0; UNII-O9XS864HTE; metaxylene; AI3-08916; M xylene; Benzene, dimethyl-; MFCD00008536; META XYLENE; 1,3-dimethyl-benzene; m-Xylene [UN1307] [Flammable liquid]; M-XYLENE [MI]; 3-XYLENE [HSDB]; bmse000554; Xylenes Reagent Grade ACS; EC 203-576-3; DSSTox_RID_76162; DSSTox_GSID_21446; m-Xylene, analytical standard; BENZENE,1,3-DIMETHYL; WLN: 1R C1; m-Xylene, anhydrous, >=99%; DTXSID6026298; m-Xylene, for synthesis, 99%; ZINC968281; m-Xylene, ReagentPlus(R), 99%; NSC61769; Tox21_200292; Tox21_202056; Tox21_303203; BDBM50008556; STL268867; m-Xylene 100 microg/mL in Methanol; AKOS000121123; NCGC00091711-01; NCGC00091711-02; NCGC00091711-03; NCGC00257052-01; NCGC00257846-01; NCGC00259605-01; m-Xylene, SAJ first grade, >=98.5%; CAS-1330-20-7; FT-0629041; S0648; X0013; EN300-24548; m-Xylene, puriss. p.a., >=99.0% (GC); C07208; J-503933; Q3234708; F1908-0174; m-Xylene, Pharmaceutical Secondary Standard; Certified Reference Material
|
|
| CAS | 108-38-3 | |
| PubChem CID | 7929 | |
| ChEMBL ID | CHEMBL286727 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 106.16 | ALogp: | 3.2 |
| HBD: | 0 | HBA: | 0 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 0.0 | Aromatic Rings: | 1 |
| Heavy Atoms: | 8 | QED Weighted: | 0.477 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.231 | MDCK Permeability: | 0.00002630 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.007 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.134 |
| 30% Bioavailability (F30%): | 0.057 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.968 | Plasma Protein Binding (PPB): | 87.88% |
| Volume Distribution (VD): | 2.228 | Fu: | 10.68% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.906 | CYP1A2-substrate: | 0.93 |
| CYP2C19-inhibitor: | 0.843 | CYP2C19-substrate: | 0.858 |
| CYP2C9-inhibitor: | 0.163 | CYP2C9-substrate: | 0.498 |
| CYP2D6-inhibitor: | 0.161 | CYP2D6-substrate: | 0.876 |
| CYP3A4-inhibitor: | 0.075 | CYP3A4-substrate: | 0.485 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 11.001 | Half-life (T1/2): | 0.754 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.034 | Human Hepatotoxicity (H-HT): | 0.112 |
| Drug-inuced Liver Injury (DILI): | 0.064 | AMES Toxicity: | 0.033 |
| Rat Oral Acute Toxicity: | 0.049 | Maximum Recommended Daily Dose: | 0.05 |
| Skin Sensitization: | 0.319 | Carcinogencity: | 0.461 |
| Eye Corrosion: | 0.98 | Eye Irritation: | 0.996 |
| Respiratory Toxicity: | 0.073 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000413 | 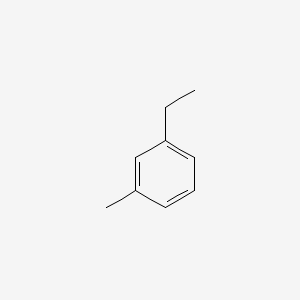 |
0.567 | D06GIP | 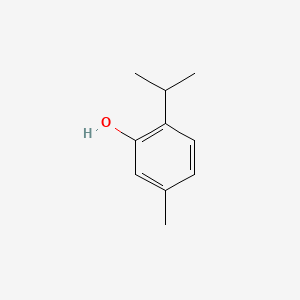 |
0.342 | ||
| ENC000368 | 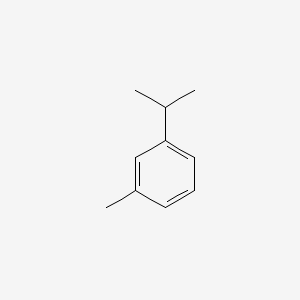 |
0.531 | D0S5LH | 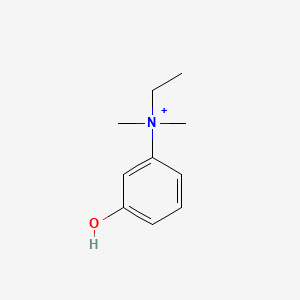 |
0.317 | ||
| ENC000414 | 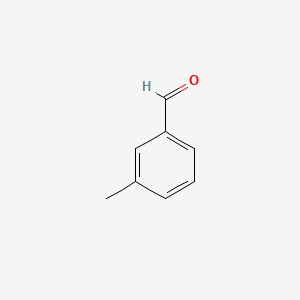 |
0.516 | D0X0RI | 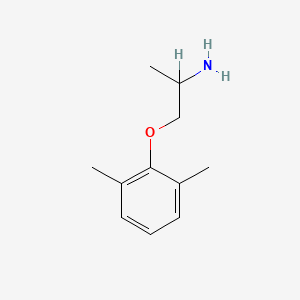 |
0.295 | ||
| ENC000370 | 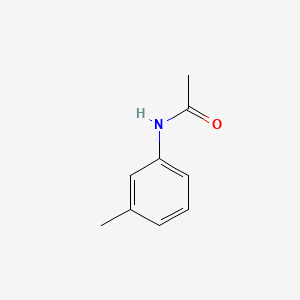 |
0.486 | D04EYC | 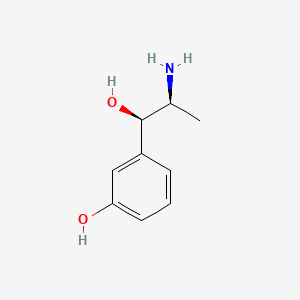 |
0.286 | ||
| ENC000233 | 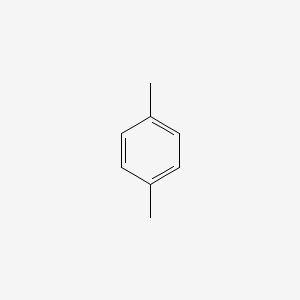 |
0.467 | D01PJR | 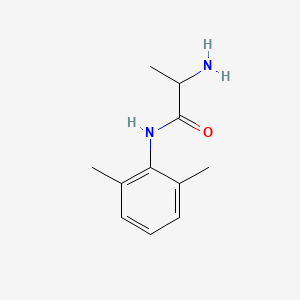 |
0.283 | ||
| ENC000064 | 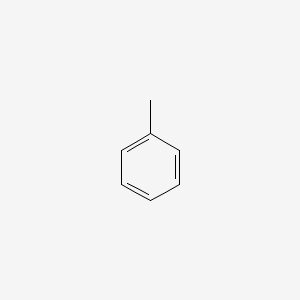 |
0.448 | D0U3DU | 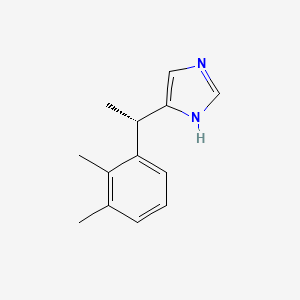 |
0.280 | ||
| ENC000180 | 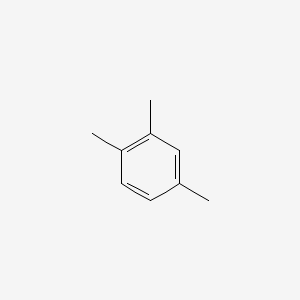 |
0.438 | D0O6IU | 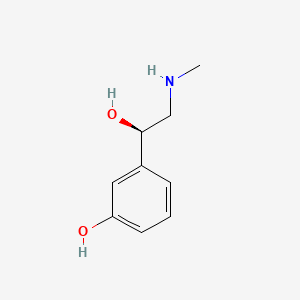 |
0.279 | ||
| ENC000392 | 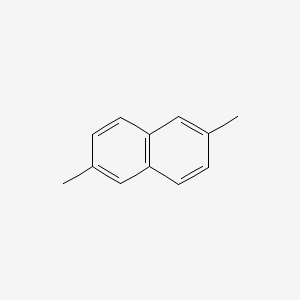 |
0.436 | D0X4ZR | 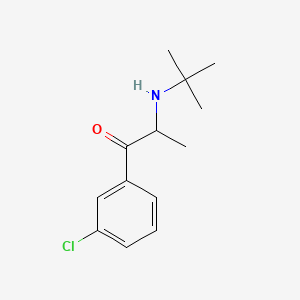 |
0.255 | ||
| ENC000179 | 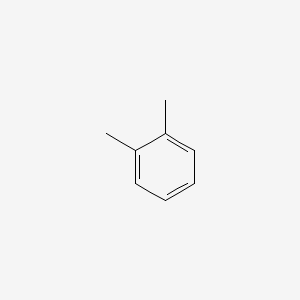 |
0.419 | D08USJ | 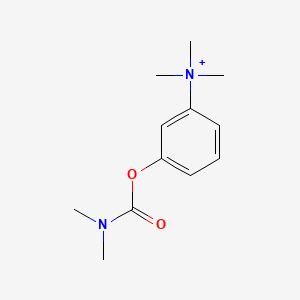 |
0.255 | ||
| ENC000240 |  |
0.419 | D0K4MH | 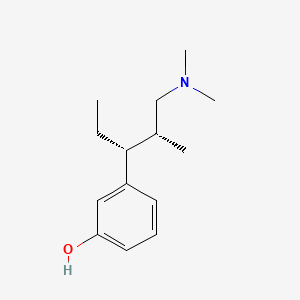 |
0.250 | ||