NPs Basic Information
|
Name |
1,2,4-Trimethylbenzene
|
| Molecular Formula | C9H12 | |
| IUPAC Name* |
1,2,4-trimethylbenzene
|
|
| SMILES |
CC1=CC(=C(C=C1)C)C
|
|
| InChI |
InChI=1S/C9H12/c1-7-4-5-8(2)9(3)6-7/h4-6H,1-3H3
|
|
| InChIKey |
GWHJZXXIDMPWGX-UHFFFAOYSA-N
|
|
| Synonyms |
1,2,4-TRIMETHYLBENZENE; Pseudocumene; 95-63-6; Pseudocumol; Psi-cumene; as-Trimethylbenzene; Benzene, 1,2,4-trimethyl-; 1,3,4-Trimethylbenzene; Uns-trimethylbenzene; 1,2,5-Trimethylbenzene; Asymmetrical trimethylbenzene; .psi.-Cumene; 1,2,4-trimethyl-benzene; Benzene, 1,2,5-trimethyl-; pseudo-cumene; 1,2,4-Trimethyl benzene; NSC 65600; CHEBI:34039; NSC-65600; 34X0W8052F; DSSTox_CID_1402; DSSTox_RID_76140; DSSTox_GSID_21402; CAS-95-63-6; 1,2,4-Trimethylbenzene, analytical standard; HSDB 5293; EINECS 202-436-9; pseudo cumene; AI3-03976; CCRIS 8146; UNII-34X0W8052F; 1,4-Trimethylbenzene; PSUEDO-CUMENE; laquo Psiraquo -Cumene; METHYL-P-XYLENE; 1,2,4 trimethylbenzene; Benzene,2,4-trimethyl-; PSEUDOCUMENE [MI]; 1,2,5-trimethyl-benzene; 1,2, 4-Trimethylbenzene; EC 202-436-9; BIDD:ER0682; TRIMETHYLBENZENE [INCI]; ScintiVerse™ II Cocktail; ScintiVerse™ LC Cocktail; 1.2.4-TRIMETHYLBENZENE; CHEMBL1797280; DTXSID6021402; WLN: 1R B1 D1; 1,2,4-Trimethylbenzene, 98%; NSC65600; TRIMETHYLBENZENE, 1,2,4-; ZINC1692473; Tox21_200518; Tox21_300049; MFCD00008527; STL268868; AKOS000120059; 1,2,4-Trimethylbenzene (pseudocumene); 1,2,4-TRIMETHYLBENZENE [HSDB]; NCGC00247891-01; NCGC00247891-02; NCGC00254118-01; NCGC00258072-01; PS-11947; 1,2,4-Trimethylbenzene (ACD/Name 4.0); FT-0606255; S0662; T0469; EN300-20076; A937622; Q376994; 1,2,4-Trimethylbenzene 100 microg/mL in Methanol; F0001-2275; Z104476700; 1,2,4-Trimethylbenzene, certified reference material, TraceCERT(R); XBZ
|
|
| CAS | 95-63-6 | |
| PubChem CID | 7247 | |
| ChEMBL ID | CHEMBL1797280 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 120.19 | ALogp: | 3.0 |
| HBD: | 0 | HBA: | 0 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 0.0 | Aromatic Rings: | 1 |
| Heavy Atoms: | 9 | QED Weighted: | 0.492 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.396 | MDCK Permeability: | 0.00002450 |
| Pgp-inhibitor: | 0.003 | Pgp-substrate: | 0.009 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.532 |
| 30% Bioavailability (F30%): | 0.872 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.936 | Plasma Protein Binding (PPB): | 90.28% |
| Volume Distribution (VD): | 1.537 | Fu: | 9.61% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.881 | CYP1A2-substrate: | 0.945 |
| CYP2C19-inhibitor: | 0.788 | CYP2C19-substrate: | 0.892 |
| CYP2C9-inhibitor: | 0.259 | CYP2C9-substrate: | 0.707 |
| CYP2D6-inhibitor: | 0.423 | CYP2D6-substrate: | 0.925 |
| CYP3A4-inhibitor: | 0.138 | CYP3A4-substrate: | 0.559 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 10.826 | Half-life (T1/2): | 0.535 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.03 | Human Hepatotoxicity (H-HT): | 0.074 |
| Drug-inuced Liver Injury (DILI): | 0.086 | AMES Toxicity: | 0.09 |
| Rat Oral Acute Toxicity: | 0.03 | Maximum Recommended Daily Dose: | 0.181 |
| Skin Sensitization: | 0.284 | Carcinogencity: | 0.623 |
| Eye Corrosion: | 0.976 | Eye Irritation: | 0.996 |
| Respiratory Toxicity: | 0.035 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
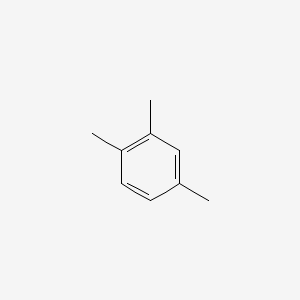
| ENC000614 |  |
0.600 | D06GIP | 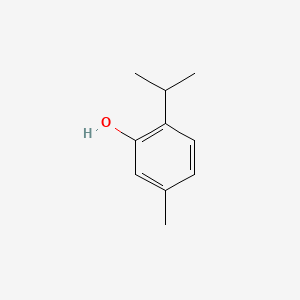 |
0.432 | ||
| ENC000498 | 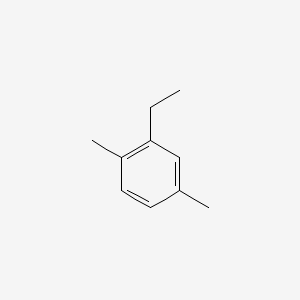 |
0.594 | D05VIX | 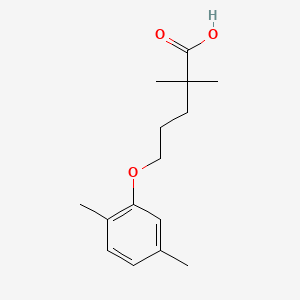 |
0.358 | ||
| ENC000552 | 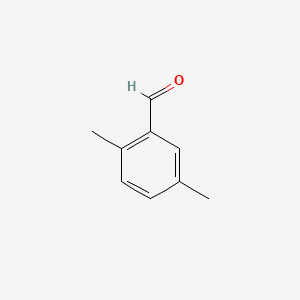 |
0.545 | D0X0RI | 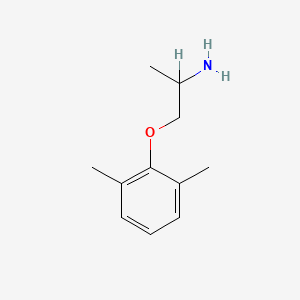 |
0.341 | ||
| ENC000649 | 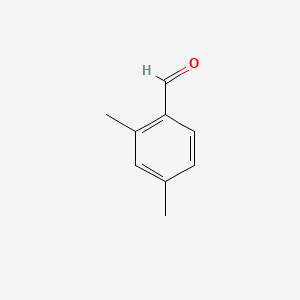 |
0.545 | D01PJR | 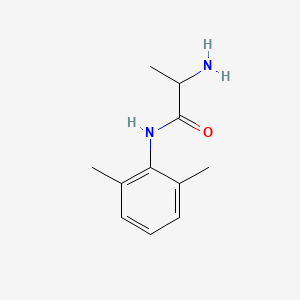 |
0.326 | ||
| ENC000364 |  |
0.500 | D0X4RN | 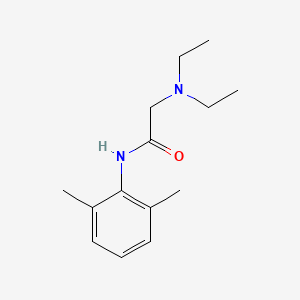 |
0.273 | ||
| ENC001744 | 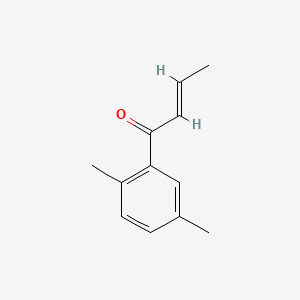 |
0.475 | D0U3DU | 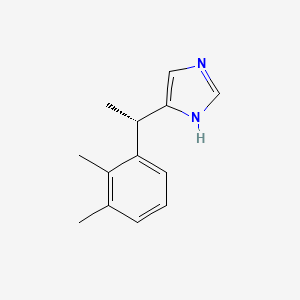 |
0.269 | ||
| ENC000342 | 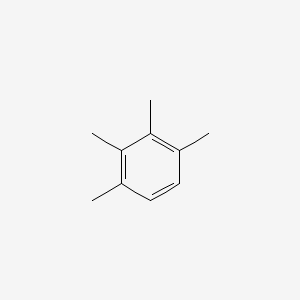 |
0.471 | D03WEX | 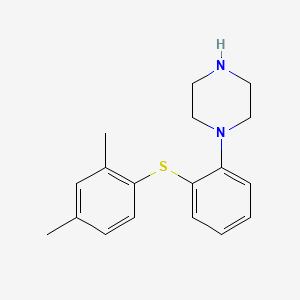 |
0.269 | ||
| ENC000239 | 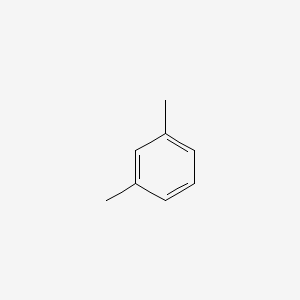 |
0.438 | D0WO8W | 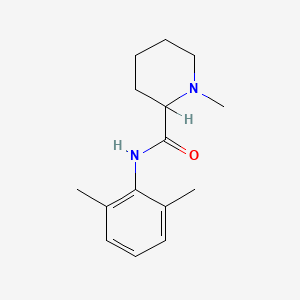 |
0.254 | ||
| ENC000179 | 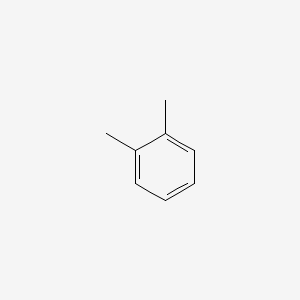 |
0.438 | D0FA2O | 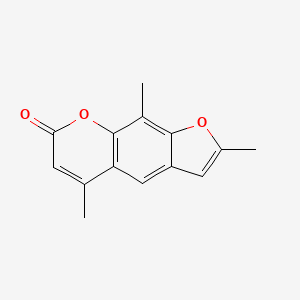 |
0.246 | ||
| ENC000181 | 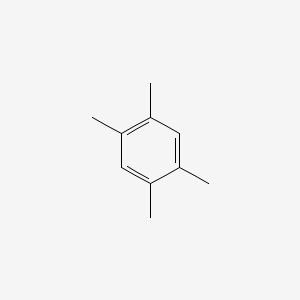 |
0.429 | D0S5LH | 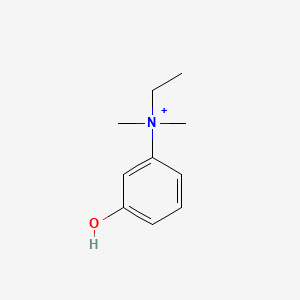 |
0.244 | ||