NPs Basic Information
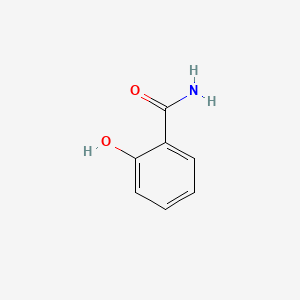
|
Name |
Salicylamide
|
| Molecular Formula | C7H7NO2 | |
| IUPAC Name* |
2-hydroxybenzamide
|
|
| SMILES |
C1=CC=C(C(=C1)C(=O)N)O
|
|
| InChI |
InChI=1S/C7H7NO2/c8-7(10)5-3-1-2-4-6(5)9/h1-4,9H,(H2,8,10)
|
|
| InChIKey |
SKZKKFZAGNVIMN-UHFFFAOYSA-N
|
|
| Synonyms |
salicylamide; 2-Hydroxybenzamide; 65-45-2; o-Hydroxybenzamide; Benzamide, 2-hydroxy-; Salicylic acid amide; 2-Carbamoylphenol; Salamide; Urtosal; 2-Carboxamidophenol; Flarpirina; Morsarinas; Algamon; Algiamida; Allevin; Amidosal; Andasol; Cetamide; Dolomide; Dropsprin; Eggosalil; Liquiprin; Novecyl; Panithal; Raspberin; Saliamid; Saliamin; Salicim; Salipur; Salizell; Salymid; Serramida; Acket; Anamid; Oramid; Salrin; Afko-Sal; Amid-Sal; Benzamide, o-hydroxy-; Salicilamida; Salicilamide; Salicylamidum; Amid kyseliny salicylove; OHB; NSC 3115; MFCD00007978; 2-Hydroxy-benzamide; H.P. 34; SR 4326; NSC-3115; EM8BM710ZC; CHEMBL27577; MLS000069486; CHEBI:32114; NCGC00091414-02; SMR000046394; Salicylamide 100 microg/mL in Acetonitrile; DSSTox_CID_1726; DSSTox_RID_76295; DSSTox_GSID_21726; Salizell (VAN); Benesal (VAN); Salicilamide [DCIT]; Salicilamide [Italian]; Salicylamidum [INN-Latin]; CAS-65-45-2; Salicilamida [INN-Spanish]; CCRIS 6045; HSDB 227; Amid kyseliny salicylove [Czech]; Benzoic acid, 2-hydroxy-, amide; EINECS 200-609-3; UNII-EM8BM710ZC; BRN 0742439; Salicylamid; Samid; AI3-03454; hydroxy benzamide; Saliclamide,(S); dihydroxybenzalamine; 2-oxidanylbenzamide; Salicylamide [USP:INN:BAN:JAN]; Salicylamide (TN); Salicylamide, 99%; Spectrum_000946; Opera_ID_1684; Spectrum2_001312; Spectrum3_000564; Spectrum4_000499; Spectrum5_001032; SALICYLAMIDE [MI]; WLN: ZVR BQ; SALICYLAMIDE [INN]; SALICYLAMIDE [JAN]; EC 200-609-3; SALICYLAMIDE [HSDB]; SALICYLAMIDE [INCI]; NCIOpen2_001127; SALICYLAMIDE [VANDF]; Oprea1_069894; SCHEMBL21646; BSPBio_001948; KBioGR_001017; KBioSS_001426; SALICYLAMIDE [MART.]; 4-10-00-00169 (Beilstein Handbook Reference); DivK1c_000858; SALICYLAMIDE [USP-RS]; SALICYLAMIDE [WHO-DD]; SPECTRUM1500532; Salicylamide (JAN/USP/INN); SPBio_001403; ZINC2055; DTXSID3021726; HMS502K20; KBio1_000858; KBio2_001426; KBio2_003994; KBio2_006562; KBio3_001448; NSC3115; NINDS_000858; HMS1920P14; HMS2092G15; HMS2232E07; Pharmakon1600-01500532; component of Tolagesic (Salt/Mix); HY-B0811; SALICYLAMIDE [USP MONOGRAPH]; Tox21_111129; Tox21_201944; Tox21_302801; BBL016007; BDBM50056900; CCG-39250; NSC757318; s6404; STK301812; AKOS000120983; Tox21_111129_1; CS-7630; DB08797; NSC-757318; IDI1_000858; NCGC00091414-01; NCGC00091414-03; NCGC00091414-04; NCGC00091414-05; NCGC00091414-07; NCGC00256376-01; NCGC00259493-01; DS-16216; Salicylamide, puriss., >=99.0% (T); SBI-0051509.P003; EU-0000058; FT-0659360; S0006; EN300-18542; D01811; AB00052089_12; A835120; AH-034/32461056; SR-01000721923; BENZOIC ACID,2-HYDROXY,AMIDE SALICYLAMIDE; J-509663; Q2496906; SR-01000721923-2; BRD-K81130846-001-02-6; BRD-K81130846-001-12-5; Z68590124; Salicylamide, United States Pharmacopeia (USP) Reference Standard; Salicylamide, Pharmaceutical Secondary Standard; Certified Reference Material
|
|
| CAS | 65-45-2 | |
| PubChem CID | 5147 | |
| ChEMBL ID | CHEMBL27577 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 137.14 | ALogp: | 1.3 |
| HBD: | 2 | HBA: | 2 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 63.3 | Aromatic Rings: | 1 |
| Heavy Atoms: | 10 | QED Weighted: | 0.603 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.577 | MDCK Permeability: | 0.00001470 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.969 |
| Human Intestinal Absorption (HIA): | 0.008 | 20% Bioavailability (F20%): | 0.012 |
| 30% Bioavailability (F30%): | 0.982 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.99 | Plasma Protein Binding (PPB): | 74.88% |
| Volume Distribution (VD): | 0.976 | Fu: | 41.65% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.415 | CYP1A2-substrate: | 0.294 |
| CYP2C19-inhibitor: | 0.066 | CYP2C19-substrate: | 0.063 |
| CYP2C9-inhibitor: | 0.055 | CYP2C9-substrate: | 0.371 |
| CYP2D6-inhibitor: | 0.152 | CYP2D6-substrate: | 0.357 |
| CYP3A4-inhibitor: | 0.015 | CYP3A4-substrate: | 0.173 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 11.683 | Half-life (T1/2): | 0.424 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.052 | Human Hepatotoxicity (H-HT): | 0.067 |
| Drug-inuced Liver Injury (DILI): | 0.574 | AMES Toxicity: | 0.061 |
| Rat Oral Acute Toxicity: | 0.094 | Maximum Recommended Daily Dose: | 0.008 |
| Skin Sensitization: | 0.187 | Carcinogencity: | 0.33 |
| Eye Corrosion: | 0.018 | Eye Irritation: | 0.969 |
| Respiratory Toxicity: | 0.42 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000104 | 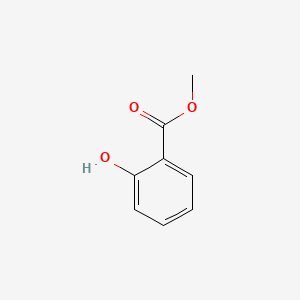 |
0.629 | D07HBX |  |
0.688 | ||
| ENC000409 | 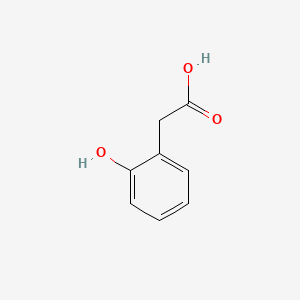 |
0.500 | D0F5ZM | 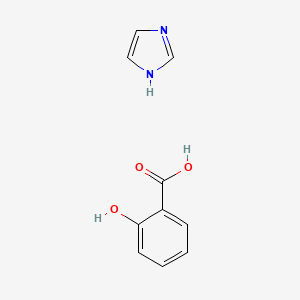 |
0.468 | ||
| ENC000166 | 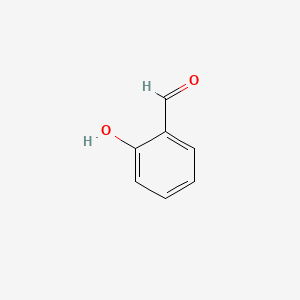 |
0.486 | D0GY5Z | 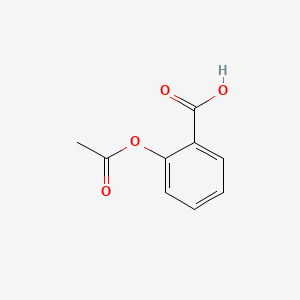 |
0.409 | ||
| ENC000076 | 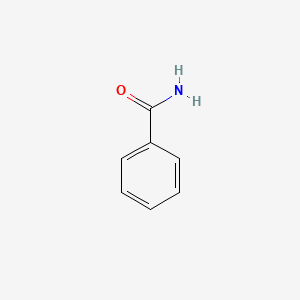 |
0.486 | D0C4YC | 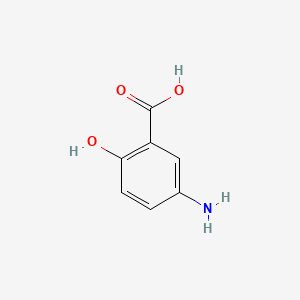 |
0.400 | ||
| ENC000021 | 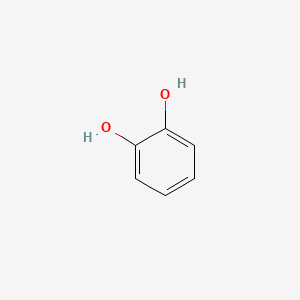 |
0.485 | D0N3UL | 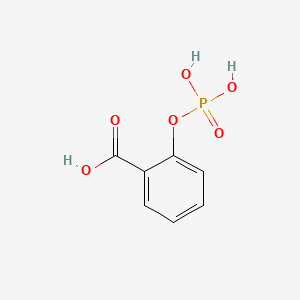 |
0.391 | ||
| ENC000028 | 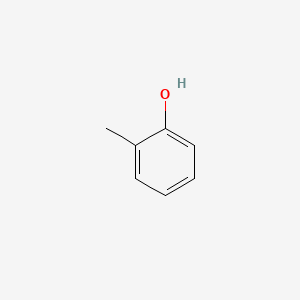 |
0.485 | D0Y0JH | 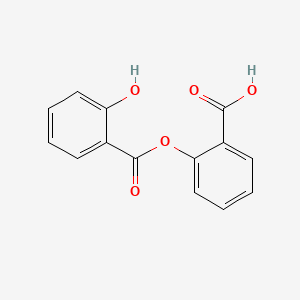 |
0.379 | ||
| ENC001547 | 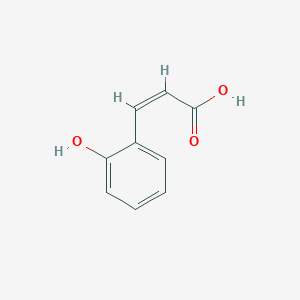 |
0.463 | D0A5CM | 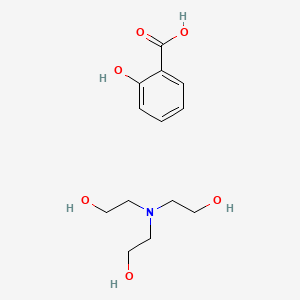 |
0.373 | ||
| ENC000303 | 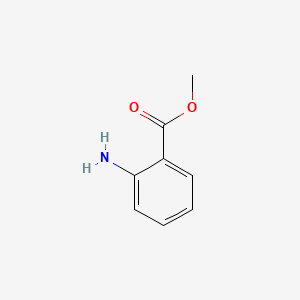 |
0.462 | D0X9RY |  |
0.368 | ||
| ENC001049 | 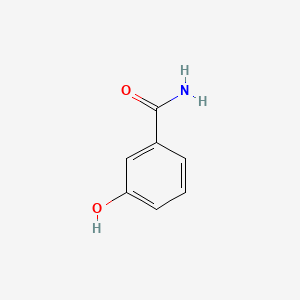 |
0.459 | D01WJL | 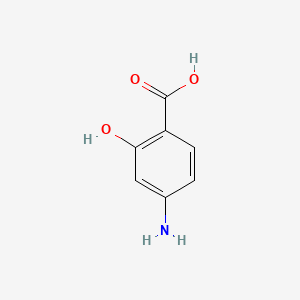 |
0.366 | ||
| ENC000033 | 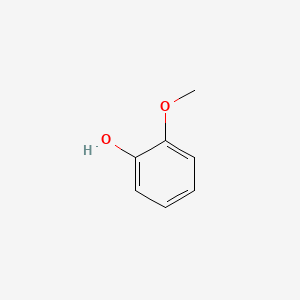 |
0.444 | D0L5PO | 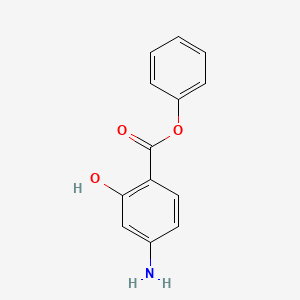 |
0.364 | ||