NPs Basic Information
|
Name |
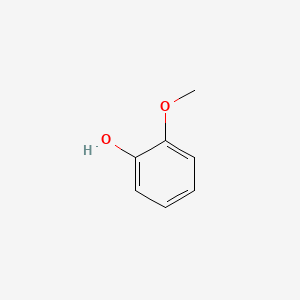
Guaiacol
|
| Molecular Formula | C7H8O2 | |
| IUPAC Name* |
2-methoxyphenol
|
|
| SMILES |
COC1=CC=CC=C1O
|
|
| InChI |
InChI=1S/C7H8O2/c1-9-7-5-3-2-4-6(7)8/h2-5,8H,1H3
|
|
| InChIKey |
LHGVFZTZFXWLCP-UHFFFAOYSA-N
|
|
| Synonyms |
guaiacol; 2-Methoxyphenol; 90-05-1; o-Methoxyphenol; 2-Hydroxyanisole; Phenol, 2-methoxy-; Guaiastil; Pyroguaiac acid; o-Guaiacol; o-Hydroxyanisole; Pyrocatechol monomethyl ether; 1-Hydroxy-2-methoxybenzene; Methylcatechol; Anastil; Guaicol; Phenol, o-methoxy-; Guaicolina; Guajol; Guasol; O-Methyl catechol; Catechol monomethyl ether; CREOSOTE, WOOD; Methoxyphenol; Guajakol; Creodon; 8021-39-4; Wood creosote; 2-Methoxy-Phenol; Hydroxyanisole; FEMA No. 2532; Guaiacol [JAN]; Methylcatachol; ortho-Guaiacol; 2-methoxy phenol; NSC 3815; (mu)-methoxyphenol; MFCD00002185; Guaiacol (JAN); Creodon (TN); NSC-3815; 2-methoxyl-4-vinylphenol; 6JKA7MAH9C; 9009-62-5; 2-methoxyphenol (guaiacol); CHEMBL13766; CHEBI:28591; Phenol, methoxy-; NCGC00090827-02; NCGC00090827-04; Guajacol; DSSTox_CID_3113; DSSTox_RID_76880; Guajakol [Czech]; DSSTox_GSID_23113; Creosote, beechwood; Guaiacol (natural); Pyrocatechol methyl ester; CAS-90-05-1; 26247-00-7; CCRIS 2943; Guaiacol [JAN:NF]; HSDB 4241; SR-01000838056; EINECS 201-964-7; UNII-6JKA7MAH9C; guiacol; Creasote; methoxy phenol; 6-methoxyphenol; hydroxyl anisole; AI3-05615; Nat.Guaiacol; O-methylcatechol; o-Guiacol; o--methoxyphenol; orthomethoxyphenol; o-methoxy-Phenol; 2-Methyloxyphenol; ortho-methoxyphenol; Guaiacol,(S); JZ3; 2-(methyloxy)phenol; GUAIACOL [FHFI]; GUAIACOL [HSDB]; GUAIACOL [MI]; GUAIACOL [VANDF]; Catechol mono methyl ether; GUAIACOL [MART.]; bmse000436; bmse010027; GUAIACOL [USP-RS]; GUAIACOL [WHO-DD]; EC 201-964-7; Guaiacol, puriss., 99%; WLN: QR BO1; DSSTox_RID_77552; 1- hydroxy-2-methoxybenzene; 3-methoxy-4-hydroxy benzene; DSSTox_GSID_24853; SCHEMBL21626; ghl.PD_Mitscher_leg0.900; guaiacol (liquid) extra pure; Guaiacol, oxidation indicator; MLS001055375; GUAIACOL [EP MONOGRAPH]; GUAIACOL [USP IMPURITY]; DTXSID0023113; NSC3815; Guaiacol, natural, >=99%, FG; HMS2089D18; HMS2233P04; HMS3372N11; HMS3715E11; Pharmakon1600-01506165; BCP27082; CS-D1347; HY-N1380; STR03604; Tox21_111031; Tox21_201136; Tox21_202990; Tox21_400004; BDBM50240369; NSC760376; s3872; STL281868; ZINC13512224; AKOS000118831; CCG-214035; DB11359; NSC-760376; PB43791; PS-3252; Guaiacol, SAJ first grade, >=98.0%; NCGC00090827-01; NCGC00090827-03; NCGC00090827-05; NCGC00090827-06; NCGC00090827-07; NCGC00258688-01; NCGC00260535-01; AC-34997; Guaiacol, Vetec(TM) reagent grade, 98%; SMR000059155; SY048708; CAS-8021-39-4; DB-024854; FT-0626815; FT-0671312; GUAIFENESIN IMPURITY A [EP IMPURITY]; M0121; EN300-19498; C01502; D00117; F70227; 2-Methoxyphenol;o-Methoxyphenol;2-Hydroxyanisole; AB00876226-06; AB00876226_07; A843426; Q412403; Q-100002; SR-01000838056-2; SR-01000838056-3; F2173-0425; Guaiacol, European Pharmacopoeia (EP) Reference Standard; Z104474028; Guaiacol, United States Pharmacopeia (USP) Reference Standard; Guaiacol, Pharmaceutical Secondary Standard; Certified Reference Material
|
|
| CAS | 90-05-1 | |
| PubChem CID | 460 | |
| ChEMBL ID | CHEMBL13766 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 124.14 | ALogp: | 1.3 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 29.5 | Aromatic Rings: | 1 |
| Heavy Atoms: | 9 | QED Weighted: | 0.619 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.337 | MDCK Permeability: | 0.00002880 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.02 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.005 |
| 30% Bioavailability (F30%): | 0.057 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.287 | Plasma Protein Binding (PPB): | 74.25% |
| Volume Distribution (VD): | 1.262 | Fu: | 18.22% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.85 | CYP1A2-substrate: | 0.937 |
| CYP2C19-inhibitor: | 0.367 | CYP2C19-substrate: | 0.789 |
| CYP2C9-inhibitor: | 0.079 | CYP2C9-substrate: | 0.849 |
| CYP2D6-inhibitor: | 0.322 | CYP2D6-substrate: | 0.881 |
| CYP3A4-inhibitor: | 0.053 | CYP3A4-substrate: | 0.324 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 13.024 | Half-life (T1/2): | 0.907 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.029 | Human Hepatotoxicity (H-HT): | 0.036 |
| Drug-inuced Liver Injury (DILI): | 0.106 | AMES Toxicity: | 0.115 |
| Rat Oral Acute Toxicity: | 0.298 | Maximum Recommended Daily Dose: | 0.019 |
| Skin Sensitization: | 0.701 | Carcinogencity: | 0.79 |
| Eye Corrosion: | 0.968 | Eye Irritation: | 0.993 |
| Respiratory Toxicity: | 0.615 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000028 | 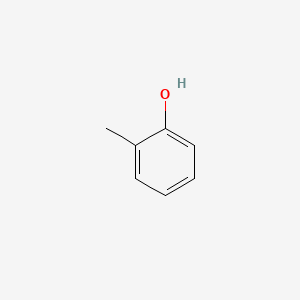 |
0.567 | D0E9CD |  |
0.447 | ||
| ENC000104 | 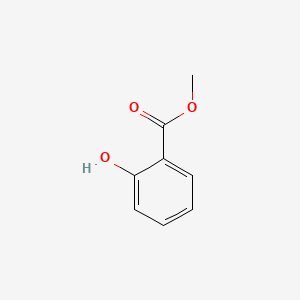 |
0.528 | D07HBX |  |
0.444 | ||
| ENC000021 | 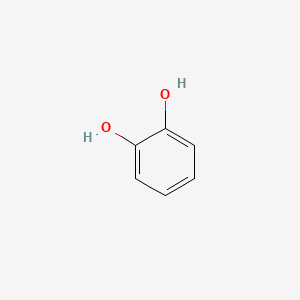 |
0.516 | D0GY5Z | 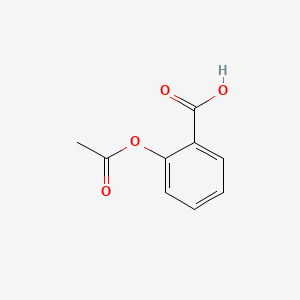 |
0.395 | ||
| ENC001367 | 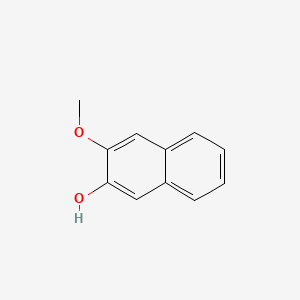 |
0.512 | D0FN7J | 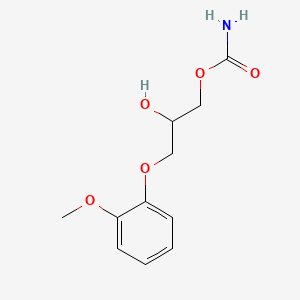 |
0.385 | ||
| ENC002077 | 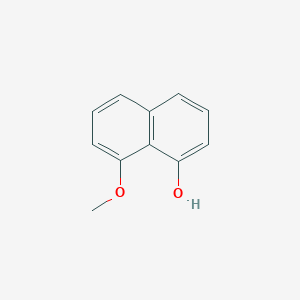 |
0.512 | D0N3UL | 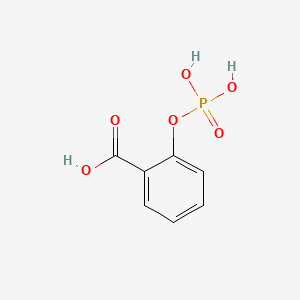 |
0.348 | ||
| ENC000172 | 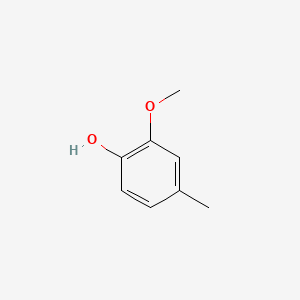 |
0.486 | D05OIS |  |
0.333 | ||
| ENC000166 | 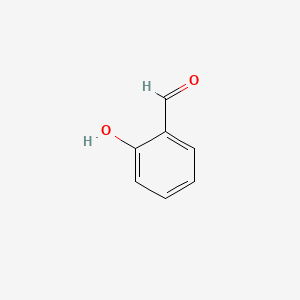 |
0.471 | D0Y0JH | 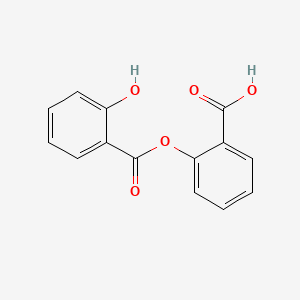 |
0.322 | ||
| ENC002213 | 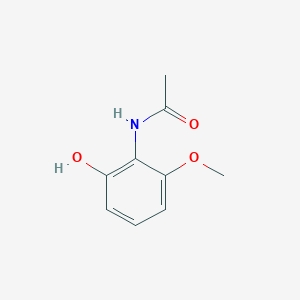 |
0.463 | D0F5ZM | 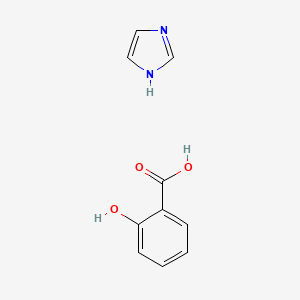 |
0.314 | ||
| ENC000207 | 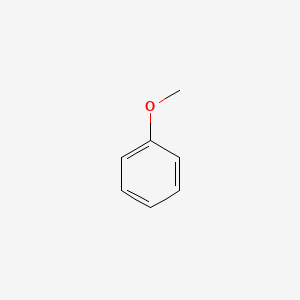 |
0.455 | D0T3LF | 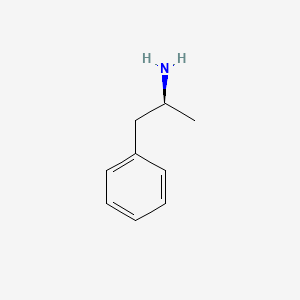 |
0.293 | ||
| ENC000823 |  |
0.452 | D05BMG |  |
0.293 | ||