NPs Basic Information
|
Name |
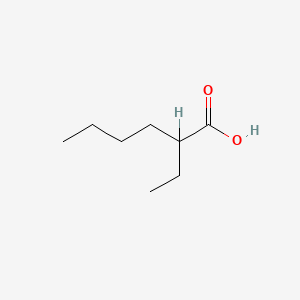
2-Ethylhexanoic acid
|
| Molecular Formula | C8H16O2 | |
| IUPAC Name* |
2-ethylhexanoic acid
|
|
| SMILES |
CCCCC(CC)C(=O)O
|
|
| InChI |
InChI=1S/C8H16O2/c1-3-5-6-7(4-2)8(9)10/h7H,3-6H2,1-2H3,(H,9,10)
|
|
| InChIKey |
OBETXYAYXDNJHR-UHFFFAOYSA-N
|
|
| Synonyms |
2-ETHYLHEXANOIC ACID; 149-57-5; 2-Ethylcaproic acid; Hexanoic acid, 2-ethyl-; Ethylhexanoic acid; Ethylhexoic acid; 2-Ethylhexoic acid; Butylethylacetic acid; 2-Butylbutanoic acid; 3-Heptanecarboxylic acid; Ethyl hexanoic acid; 2-ethyl-hexoic acid; 2-ethyl hexanoic acid; alpha-Ethylcaproic acid; 2-ethyl-hexanoic acid; Ethyl hexanoic acid, 2-; 2 ETHYL HEXANOIC ACID; alpha-ethyl caproic acid; .alpha.-Ethylcaproic acid; 2-Ethyl-1-hexanoic acid; (+/-)-2-ETHYLHEXANOIC ACID; 01MU2J7VVZ; 2-ETHYL HEXOIC ACID,AR; 61788-37-2; CHEBI:89058; NSC-8881; 2-ethylhexanoicacid; DSSTox_CID_5293; 2-Ethylhexansaeure; DSSTox_RID_77730; DSSTox_GSID_25293; 2-Ethylhexanoic acid, >=99%; 2-Ethylhexanoic acid, analytical standard; CAS-149-57-5; CCRIS 3348; HSDB 5649; Kyselina 2-ethylkapronova [Czech]; NSC 8881; Kyselina 2-ethylkapronova; EINECS 205-743-6; UNII-01MU2J7VVZ; Kyselina heptan-3-karboxylova [Czech]; BRN 1750468; Kyselina heptan-3-karboxylova; AI3-01371; Hexanoic acid, 2-ethyl-, (-)-; EINECS 262-971-9; MFCD00002675; 2-Ethylcapronic acid; 2-Ethyl-Hexonic acid; alpha-Ethylhexanoic acid; .alpha.-Ethylhexanoic acid; EC 205-743-6; SCHEMBL25800; 2-Ethylhexanoic acid, 99%; MLS002415695; CHEMBL1162485; DTXSID9025293; WLN: QVY4 & 2; NSC8881; HMS2267F21; STR05759; 2-ETHYLHEXANOIC ACID [HSDB]; Tox21_201406; Tox21_300108; LMFA01020087; AKOS009031416; AT29893; CS-W016381; SB44987; SB44994; Hexanoic acid,2-ethyl-, tridecyl ester; NCGC00091324-01; NCGC00091324-02; NCGC00091324-03; NCGC00253985-01; NCGC00258957-01; SMR001252268; E0120; FT-0612273; FT-0654390; EN300-20410; Q209384; W-109079; F0001-0703; Z104478072; 18FEB650-7573-4EA0-B0CD-9D8BED766547; 2-Ethylhexanoic acid, Pharmaceutical Secondary Standard; Certified Reference Material
|
|
| CAS | 149-57-5 | |
| PubChem CID | 8697 | |
| ChEMBL ID | CHEMBL1162485 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 144.21 | ALogp: | 2.6 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 5 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 37.3 | Aromatic Rings: | 0 |
| Heavy Atoms: | 10 | QED Weighted: | 0.644 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.443 | MDCK Permeability: | 0.00002870 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.006 | 20% Bioavailability (F20%): | 0.002 |
| 30% Bioavailability (F30%): | 0.022 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.959 | Plasma Protein Binding (PPB): | 80.97% |
| Volume Distribution (VD): | 0.376 | Fu: | 16.17% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.059 | CYP1A2-substrate: | 0.771 |
| CYP2C19-inhibitor: | 0.027 | CYP2C19-substrate: | 0.813 |
| CYP2C9-inhibitor: | 0.029 | CYP2C9-substrate: | 0.938 |
| CYP2D6-inhibitor: | 0.011 | CYP2D6-substrate: | 0.226 |
| CYP3A4-inhibitor: | 0.007 | CYP3A4-substrate: | 0.06 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 3.657 | Half-life (T1/2): | 0.789 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.012 | Human Hepatotoxicity (H-HT): | 0.106 |
| Drug-inuced Liver Injury (DILI): | 0.03 | AMES Toxicity: | 0.006 |
| Rat Oral Acute Toxicity: | 0.063 | Maximum Recommended Daily Dose: | 0.019 |
| Skin Sensitization: | 0.221 | Carcinogencity: | 0.131 |
| Eye Corrosion: | 0.98 | Eye Irritation: | 0.991 |
| Respiratory Toxicity: | 0.404 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000833 | 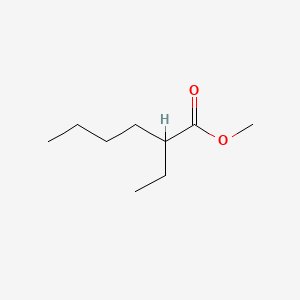 |
0.618 | D0Y3KG | 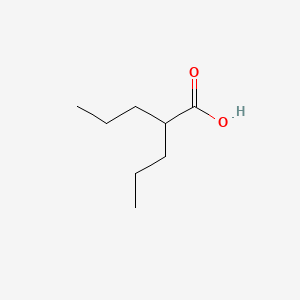 |
0.576 | ||
| ENC000652 | 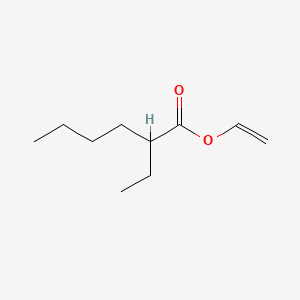 |
0.568 | D03LGY | 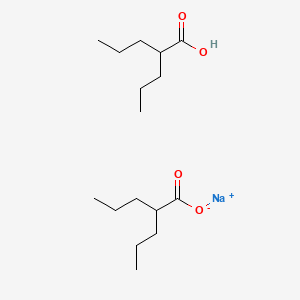 |
0.317 | ||
| ENC000550 | 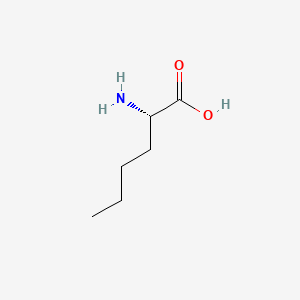 |
0.531 | D01OPV | 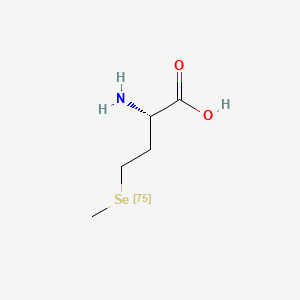 |
0.289 | ||
| ENC002444 | 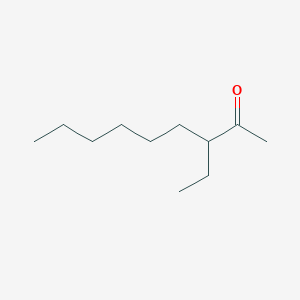 |
0.526 | D03CHT | 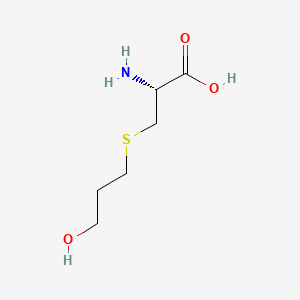 |
0.279 | ||
| ENC001899 | 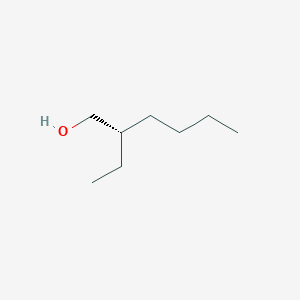 |
0.471 | D0A5JP | 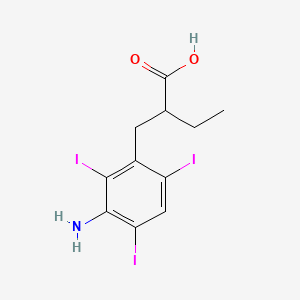 |
0.273 | ||
| ENC000220 | 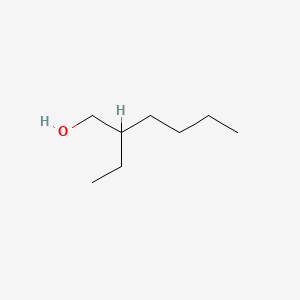 |
0.471 | D0F5DO | 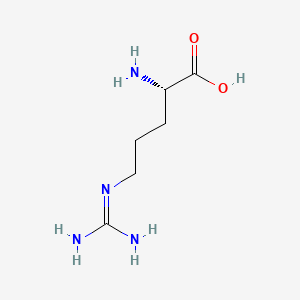 |
0.267 | ||
| ENC000889 | 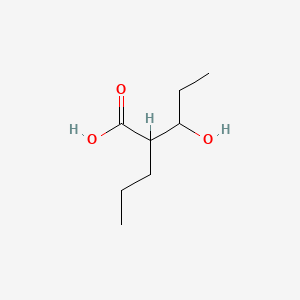 |
0.459 | D01QLH | 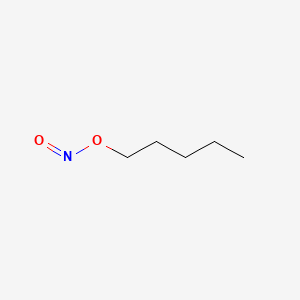 |
0.263 | ||
| ENC000890 | 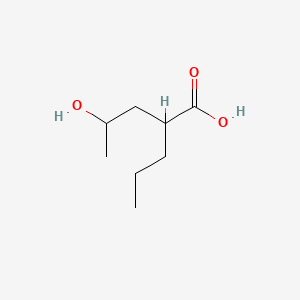 |
0.459 | D09PUL | 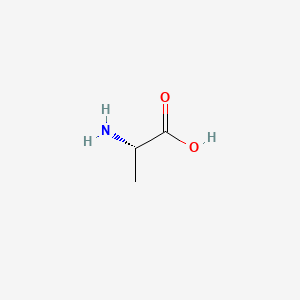 |
0.258 | ||
| ENC000211 | 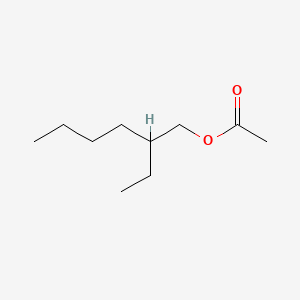 |
0.450 | D0EP8X | 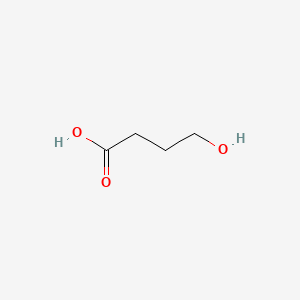 |
0.257 | ||
| ENC000398 | 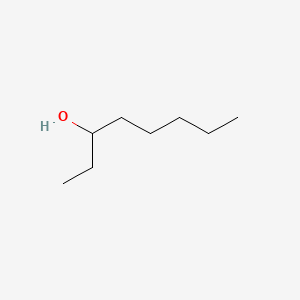 |
0.429 | D00WUF |  |
0.256 | ||