NPs Basic Information
|
Name |
Thymine
|
| Molecular Formula | C5H6N2O2 | |
| IUPAC Name* |
5-methyl-1H-pyrimidine-2,4-dione
|
|
| SMILES |
CC1=CNC(=O)NC1=O
|
|
| InChI |
InChI=1S/C5H6N2O2/c1-3-2-6-5(9)7-4(3)8/h2H,1H3,(H2,6,7,8,9)
|
|
| InChIKey |
RWQNBRDOKXIBIV-UHFFFAOYSA-N
|
|
| Synonyms |
thymine; 5-methyluracil; 65-71-4; 2,4-Dihydroxy-5-methylpyrimidine; Thymin; 5-methylpyrimidine-2,4(1H,3H)-dione; Thymine anhydrate; 2,4(1H,3H)-Pyrimidinedione, 5-methyl-; 5-methyl-2,4(1H,3H)-pyrimidinedione; Thymin (purine base); 5-methyl-1H-pyrimidine-2,4-dione; 5-Methyl-1,2,3,4-tetrahydropyrimidine-2,4-dione; 5-Methyl Uracil; CCRIS 5584; 5-methylpyrimidine-2,4-diol; 5-Methylpyrimidine-2,4-dione; 4-Hydroxy-5-methylpyrimidin-2(1H)-one; CHEBI:17821; AI3-25479; NSC14705; MFCD00006026; QR26YLT7LT; CHEMBL993; 5-Methyl-2,4-dihydroxypyrimidine; NSC-14705; 123430-67-1; 153445-43-3; 5-METHYLPYRIMIDINE-2,4(1H,3H)-DIONE (THYMINE); Thy; Thymine-t; Thymine (VAN); (3H)Methylthymidine; Methyl-3H thymidine; Thymine (8CI); NSC-168663; 5-methy uracil(Thymine); EINECS 200-616-1; UNII-QR26YLT7LT; NSC 14705; 5-methyl-uracil; Thymine,(S); THYMINE [INCI]; Thymine, >=99%; THYMINE [MI]; Zidovudine EP Impurity C; THYMINE [WHO-DD]; Epitope ID:167476; SCHEMBL5235; DSSTox_CID_30914; DSSTox_GSID_52342; 2,4(1H,3H)-Pyrimidinedione, 5-methyl- (9CI); 4(1H)-Pyrimidinone, 2-hydroxy-5-methyl- (9CI); 4(3H)-Pyrimidinone, 2-hydroxy-5-methyl- (9CI); 5-Methyl-2,4-dioxypyrimidine; THYMINE [USP IMPURITY]; 2.6-Dioxy-5-methyl-pyrimidin; GTPL4581; DTXSID4052342; SCHEMBL15496760; SCHEMBL16356870; WLN: T6N CNJ BQ DQ E1; 2,4(1H,3H)-Pyrimidinedione, 5-methyl-, labeled with tritium; ZINC157062; BCP22973; 2,3H)-Pyrimidinedione, 5-methyl-; Tox21_303929; BDBM50134397; s9382; STL280241; STL477641; STAVUINE IMPURITY A [WHO-IP]; AKOS000120923; AKOS002337369; AC-7756; AM81332; CCG-266101; CS-W011166; DB03462; HY-W010450; SB57778; ZIDOVUDINE IMPURITY C [WHO-IP]; CAS-65-71-4; CID 5274265; NCGC00357169-01; 2792-47-4; SY014896; Thymine, Vetec(TM) reagent grade, 99%; DB-016098; ZIDOVUDINE IMPURITY C [EP IMPURITY]; FT-0602550; FT-0675207; FT-0771534; T0234; EN300-21969; C00178; T-3845; Thymine, suitable for cell culture, BioReagent; 006T026; A835203; Q171973; SR-01000945223; SR-01000945223-1; 036B9F1D-9B61-4CED-967C-BF1DA180E5C2; F0001-1753; Z147641104; 3059-73-2
|
|
| CAS | 65-71-4 | |
| PubChem CID | 1135 | |
| ChEMBL ID | CHEMBL993 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 126.11 | ALogp: | -0.6 |
| HBD: | 2 | HBA: | 2 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 58.2 | Aromatic Rings: | 1 |
| Heavy Atoms: | 9 | QED Weighted: | 0.506 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.064 | MDCK Permeability: | 0.00001310 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.178 |
| Human Intestinal Absorption (HIA): | 0.968 | 20% Bioavailability (F20%): | 0.01 |
| 30% Bioavailability (F30%): | 0.845 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.051 | Plasma Protein Binding (PPB): | 12.67% |
| Volume Distribution (VD): | 0.425 | Fu: | 78.42% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.046 | CYP1A2-substrate: | 0.973 |
| CYP2C19-inhibitor: | 0.083 | CYP2C19-substrate: | 0.057 |
| CYP2C9-inhibitor: | 0.013 | CYP2C9-substrate: | 0.156 |
| CYP2D6-inhibitor: | 0.008 | CYP2D6-substrate: | 0.046 |
| CYP3A4-inhibitor: | 0.009 | CYP3A4-substrate: | 0.243 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.909 | Half-life (T1/2): | 0.945 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.018 | Human Hepatotoxicity (H-HT): | 0.696 |
| Drug-inuced Liver Injury (DILI): | 0.97 | AMES Toxicity: | 0.021 |
| Rat Oral Acute Toxicity: | 0.117 | Maximum Recommended Daily Dose: | 0.015 |
| Skin Sensitization: | 0.301 | Carcinogencity: | 0.132 |
| Eye Corrosion: | 0.008 | Eye Irritation: | 0.971 |
| Respiratory Toxicity: | 0.017 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
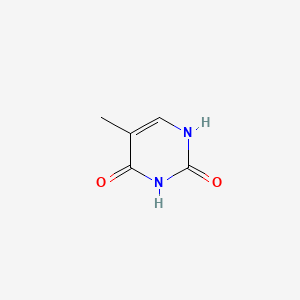
| ENC000065 | 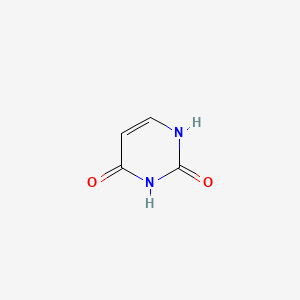 |
0.394 | D05LEO | 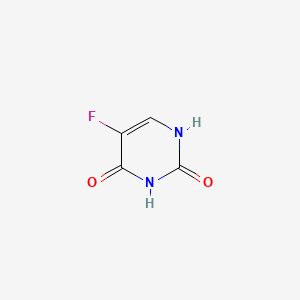 |
0.600 | ||
| ENC001638 | 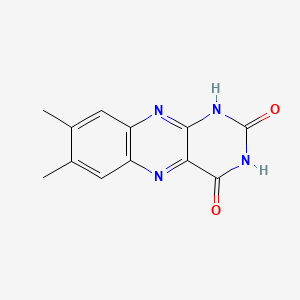 |
0.298 | D0J9UN | 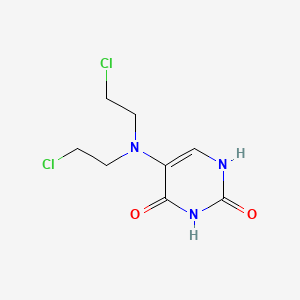 |
0.383 | ||
| ENC000066 | 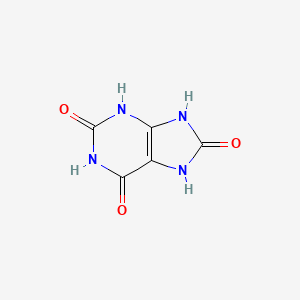 |
0.295 | D09AMZ | 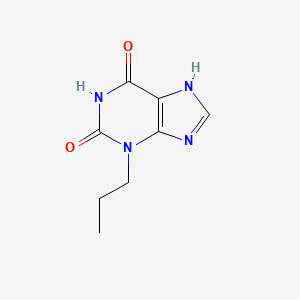 |
0.286 | ||
| ENC000120 | 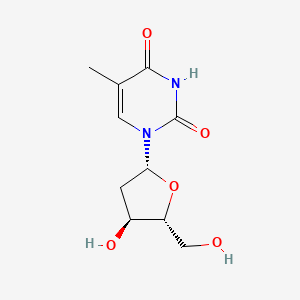 |
0.273 | D0Z8EX | 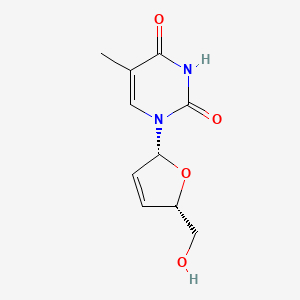 |
0.283 | ||
| ENC003235 | 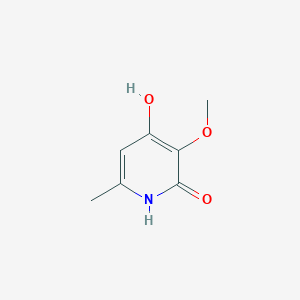 |
0.262 | D0CL9S | 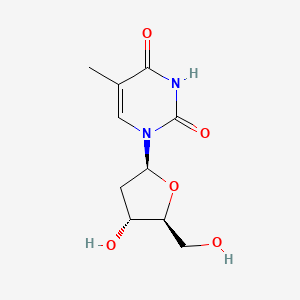 |
0.273 | ||
| ENC001061 | 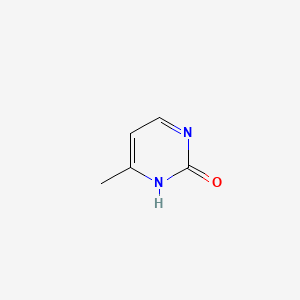 |
0.243 | D0I0DS | 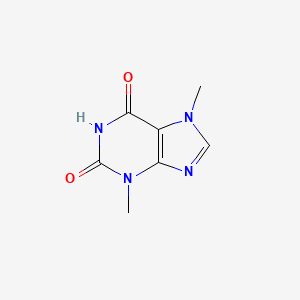 |
0.255 | ||
| ENC003436 | 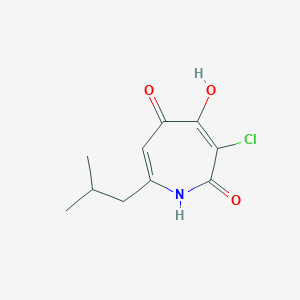 |
0.235 | D01XYJ | 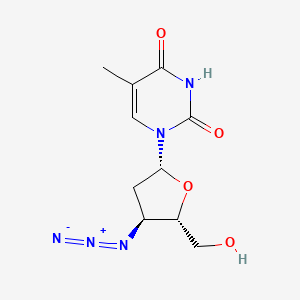 |
0.246 | ||
| ENC002826 | 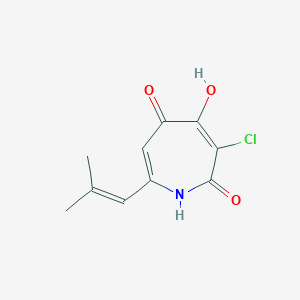 |
0.235 | D04KYY | 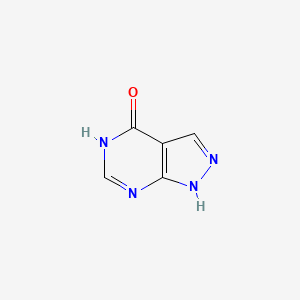 |
0.233 | ||
| ENC000721 | 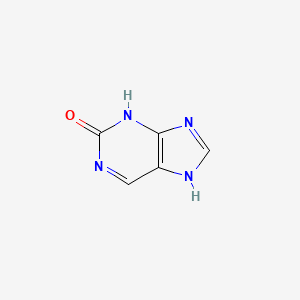 |
0.233 | D0WB9V | 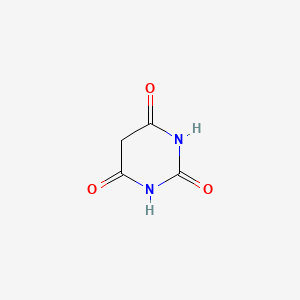 |
0.231 | ||
| ENC005486 | 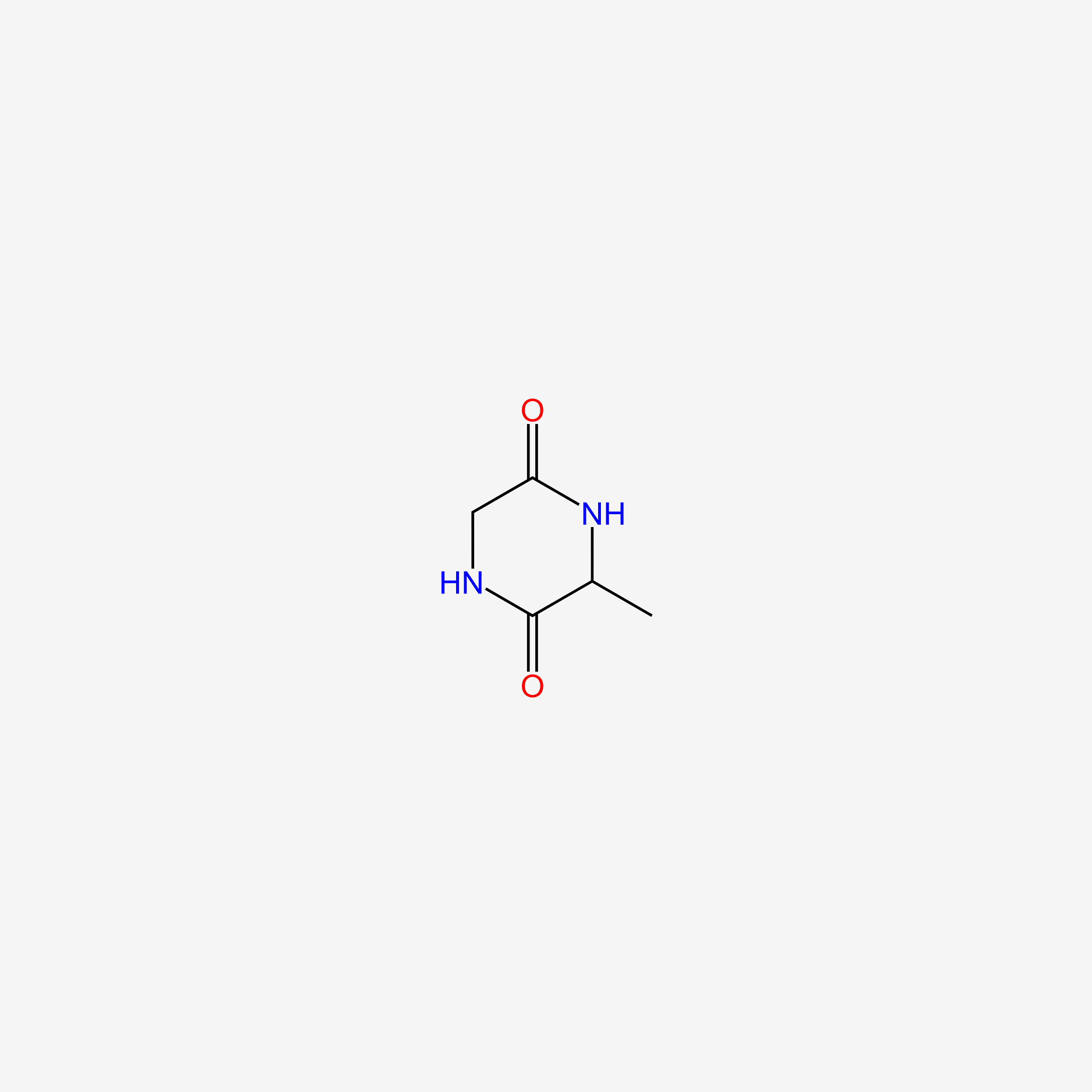 |
0.231 | D0F8RA | 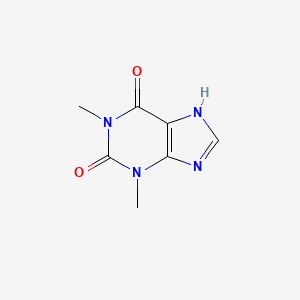 |
0.229 | ||