NPs Basic Information
|
Name |
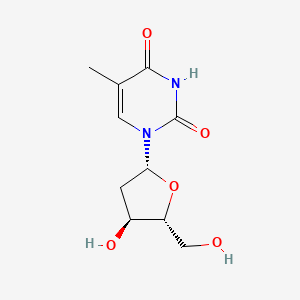
Thymidine
|
| Molecular Formula | C10H14N2O5 | |
| IUPAC Name* |
1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-methylpyrimidine-2,4-dione
|
|
| SMILES |
CC1=CN(C(=O)NC1=O)[C@H]2C[C@@H]([C@H](O2)CO)O
|
|
| InChI |
InChI=1S/C10H14N2O5/c1-5-3-12(10(16)11-9(5)15)8-2-6(14)7(4-13)17-8/h3,6-8,13-14H,2,4H2,1H3,(H,11,15,16)/t6-,7+,8+/m0/s1
|
|
| InChIKey |
IQFYYKKMVGJFEH-XLPZGREQSA-N
|
|
| Synonyms |
thymidine; 50-89-5; deoxythymidine; 2'-Deoxythymidine; Thymidin; 5-Methyldeoxyuridine; Beta-Thymidine; DThyd; 5-Methyl-2'-deoxyuridine; Thymine-2-desoxyriboside; 5-Methyldeoxyurindine; Deoxyribothymidine; Thymine-2-deoxyriboside; Thyminedeoxyriboside; Thymine deoxyriboside; dThd; 2'-thymidine; Uridine, 2'-deoxy-5-methyl-; dT; Doxribtimine; thymine 2'-deoxyriboside; 2'-deoxy-5-methyluridine; AI3-52267; beta-D-Ribofuranoside, thymine-1 2-deoxy-; 157049-39-3; NSC 21548; 1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-methylpyrimidine-2,4-dione; CHEBI:17748; 1-(2-Deoxy-beta-D-erythro-pentofuranosyl)-5-methyl-2,4(1H,3H)-pyrimidinedione; 2,4(1H,3H)-Pyrimidinedione, 1-(2-deoxy-beta-D-erythro-pentofuranosyl)-5-methyl-; Thymine 2-desoxyriboside; VC2W18DGKR; 1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-methyl-1,2,3,4-tetrahydropyrimidine-2,4-dione; 1-((2R,4S,5R)-4-Hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-methylpyrimidine-2,4(1H,3H)-dione; NSC-21548; [3H]-Thymidine; NCGC00142484-03; MFCD00006537; alpha-tritiated thymidine; 146183-25-7; 157049-40-6; THM; 1-(2-deoxy-beta-D-erythro-pentofuranosyl)-5-methylpyrimidine-2,4(1H,3H)-dione; 1-(2-Deoxy-beta-D-erythro-pentofuranosyl)-5-methylpyrimidine-2,4(1H,3H)-dione (Thymidine); 1-((2R,4S,5R)-4-Hydroxy-5-(hydroxymethyl)tetrahydro-furan-2-yl)-5-methylpyrimidine-2,4(1H,3H)-dione; CCRIS 1283; EINECS 200-070-4; UNII-VC2W18DGKR; deoxyribosylthymine; 4qsv; Thymidinedeoxyriboside; 1w2g; Thymidine, >=99%; BDBM1; THYMIDINE [MI]; THYMIDINE [INCI]; Thymidine (8CI,9CI); DSSTox_CID_3661; DOXRIBTIMINE [INN]; bmse000244; Epitope ID:138113; THYMIDINE [MART.]; DOXRIBTIMINE [USAN]; EC 200-070-4; THYMIDINE [WHO-DD]; THYMIDINE [WHO-IP]; 2'-deoxy-5-methyl-Uridine; DSSTox_RID_77133; DSSTox_GSID_23661; SCHEMBL19894; 50-88-4; 2'-dT; CHEMBL52609; GTPL4718; DTXSID5023661; ZINC25672; IMPURITY C [EP IMPURITY]; ACT03217; HY-N1150; STR05630; Thymidine, >=99.0% (HPLC); Tox21_111560; HG1139; s4803; AKOS015895360; STAVUDINE IMPURITY C [WHO-IP]; thymine-1 2-deoxy-b-D-Ribofuranoside; AC-1475; AM83949; CCG-266886; CS-W019644; DB04485; CAS-50-89-5; DEOXYTHYMIDINE; 2'-DEOXYTHYMIDINE; NCGC00142484-01; 1-[(4S,2R,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-methyl-1,3-dihydropyri midine-2,4-dione; STAVUDINE IMPURITY C [EP IMPURITY]; Thymidine, Vetec(TM) reagent grade, 99%; DB-030432; ZIDOVUDINE IMPURITY E [EP IMPURITY]; T0233; thymine-1 2-deoxy-beta-delta-Ribofuranoside; MT-1621 COMPONENT 2'-DEOXYTHYMIDINE; 1-(2-Deoxy-A-D-ribofuranosyl)-5-methyluracil; 1-(2-Deoxy-beta-ribofuranosyl)-5-methyluracil; C00214; EN300-378408; A828337; Q422464; SR-01000883981; (1-[2-Deoxy-beta-D-ribofuranosyl]-5-methyluracil); 1-(2-Deoxy-.beta.-D-ribofuranosyl)-5-methyluracil; J-700251; SR-01000883981-1; BRD-K28309349-001-02-4; Q27124084; D7E43A69-265B-4DAA-BC8F-193FB357140F; Thymidine, powder, BioReagent, suitable for cell culture; Z1741976665; 1-[4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-methyl-pyrimidine-2,4-dione; 1-(2-Deoxy-b-D-erythro-pentofuranosyl)-5-methyl-2,4(1H,3H)-pyrimidinedione; 1-(2-Deoxy-beta-delta-erythro-pentofuranosyl)-5-methyl-2,4(1H,3H)-pyrimidinedione; 1-[(2R,4S,5R)-4-Hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-methyl-pyrimidine-2,4-dione; 1-(2-DEOXY-.BETA.-D-ERYTHRO-PENTOFURANOSYL)-5-METHYLPYRIMIDINE-2,4(1H,3H)-DIONE [WHO-IP]; 1-(2-Deoxy-beta-D-ribofuranosyl)-5-methyluracil; 1-(2-Deoxy-beta-D-ribofuranosyl)thymine; Thymine deoxyriboside; 2'-Deoxythymidine; 5-Methyldeoxyuridine; 1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-5-methyl-pyrimidine-2,4-dione; 1211376-53-2
|
|
| CAS | 146183-25-7 | |
| PubChem CID | 5789 | |
| ChEMBL ID | CHEMBL52609 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 242.23 | ALogp: | -1.2 |
| HBD: | 3 | HBA: | 5 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 99.1 | Aromatic Rings: | 2 |
| Heavy Atoms: | 17 | QED Weighted: | 0.619 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.755 | MDCK Permeability: | 0.00017833 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.145 |
| Human Intestinal Absorption (HIA): | 0.019 | 20% Bioavailability (F20%): | 0.01 |
| 30% Bioavailability (F30%): | 0.016 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.384 | Plasma Protein Binding (PPB): | 11.86% |
| Volume Distribution (VD): | 0.563 | Fu: | 78.45% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.019 | CYP1A2-substrate: | 0.218 |
| CYP2C19-inhibitor: | 0.028 | CYP2C19-substrate: | 0.06 |
| CYP2C9-inhibitor: | 0.003 | CYP2C9-substrate: | 0.365 |
| CYP2D6-inhibitor: | 0.008 | CYP2D6-substrate: | 0.109 |
| CYP3A4-inhibitor: | 0.005 | CYP3A4-substrate: | 0.162 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 8.901 | Half-life (T1/2): | 0.906 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.012 | Human Hepatotoxicity (H-HT): | 0.948 |
| Drug-inuced Liver Injury (DILI): | 0.984 | AMES Toxicity: | 0.228 |
| Rat Oral Acute Toxicity: | 0.008 | Maximum Recommended Daily Dose: | 0.074 |
| Skin Sensitization: | 0.102 | Carcinogencity: | 0.118 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.023 |
| Respiratory Toxicity: | 0.01 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000126 | 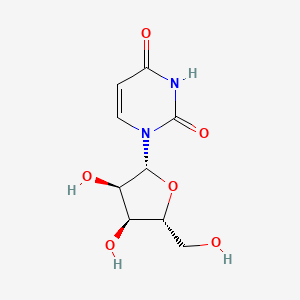 |
0.394 | D0CL9S | 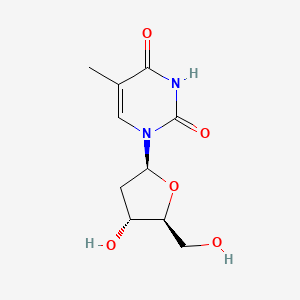 |
1.000 | ||
| ENC001221 |  |
0.339 | D09PZO | 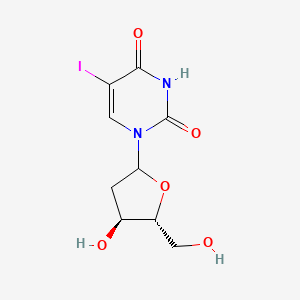 |
0.769 | ||
| ENC005638 | 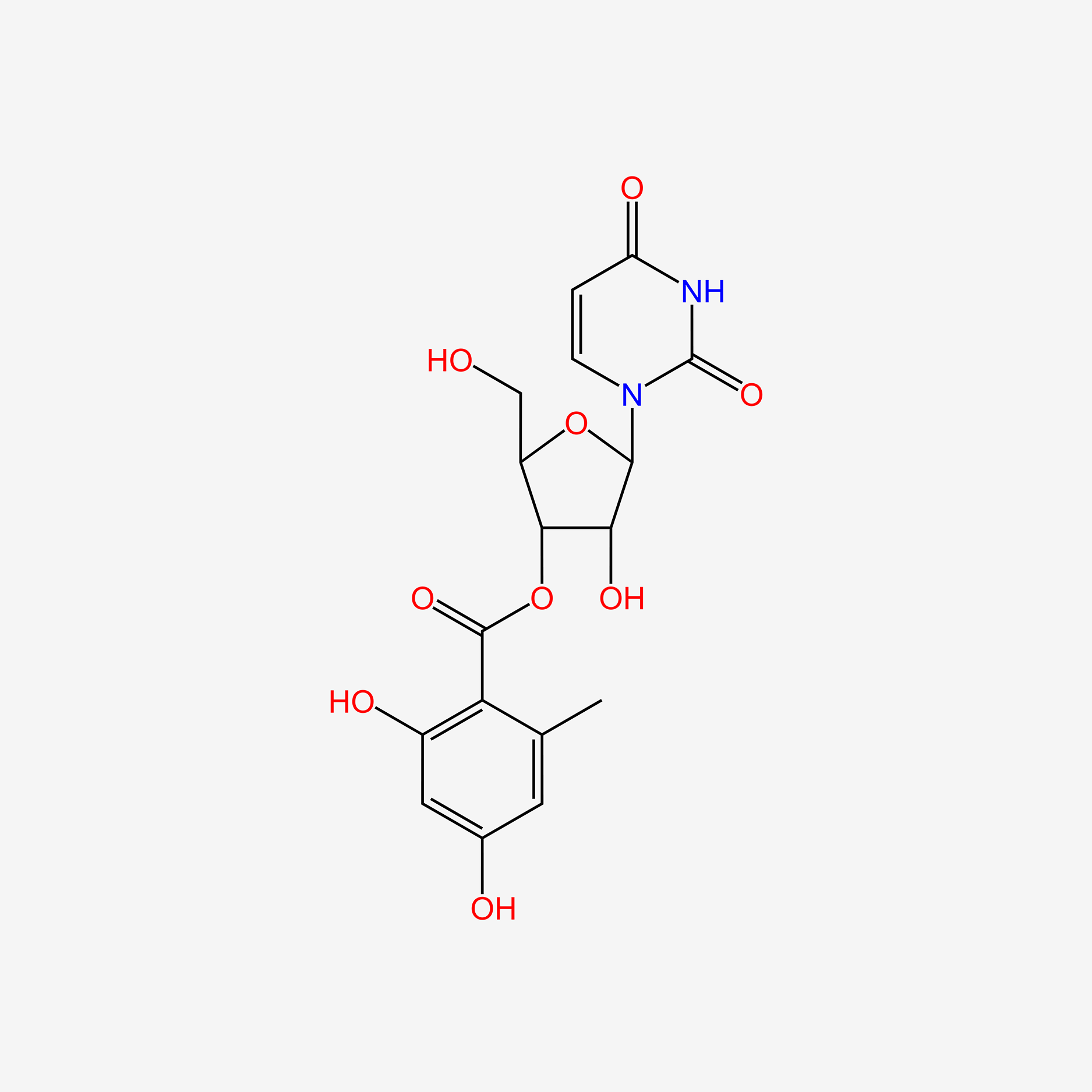 |
0.326 | D0TS1Z | 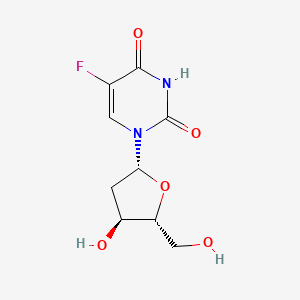 |
0.769 | ||
| ENC005639 | 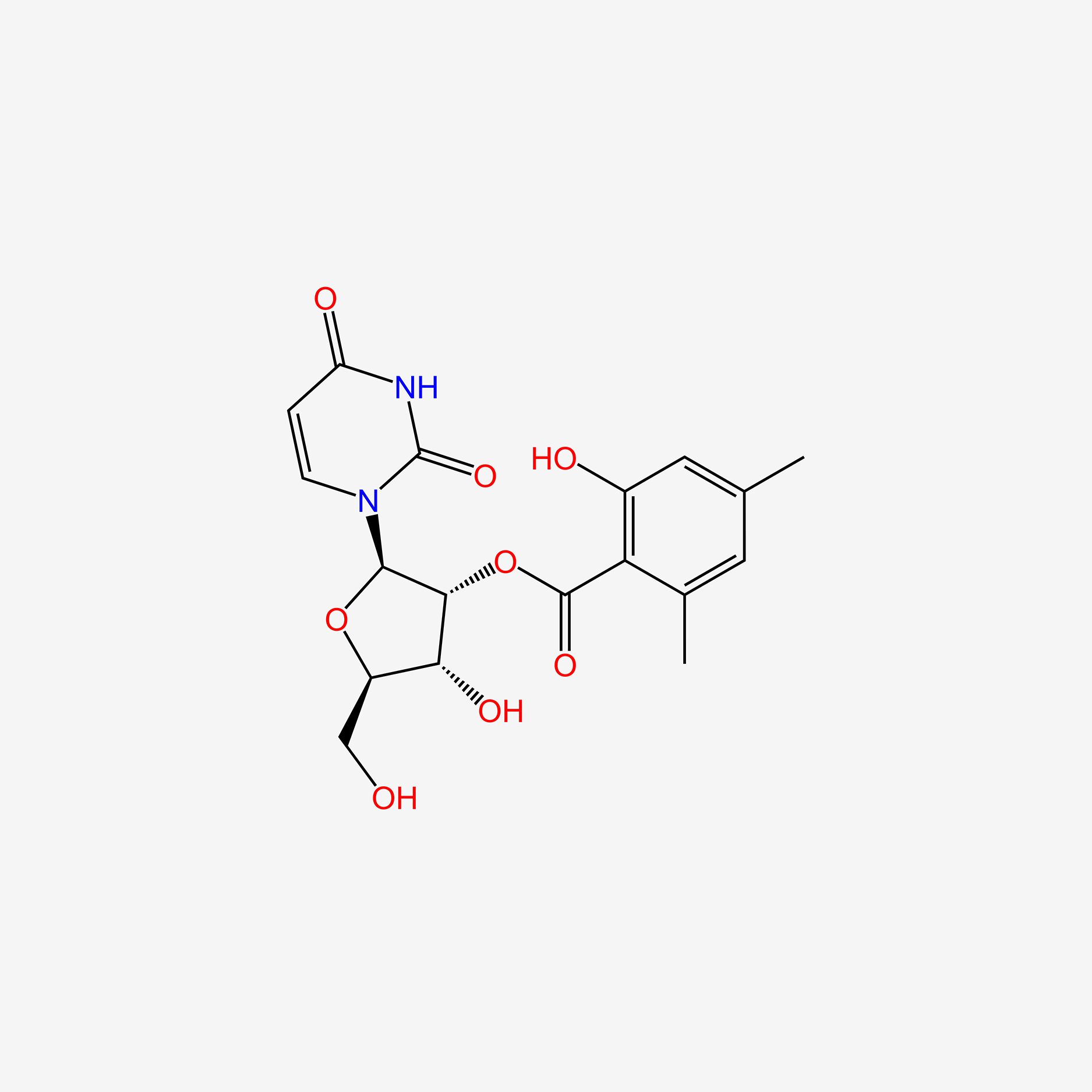 |
0.326 | D0R2KF | 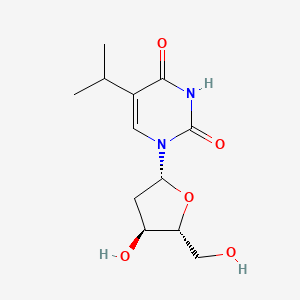 |
0.732 | ||
| ENC002576 | 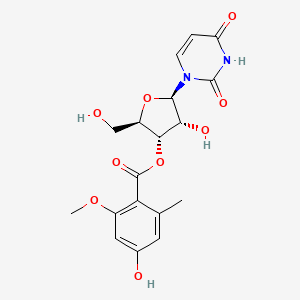 |
0.316 | D01XYJ | 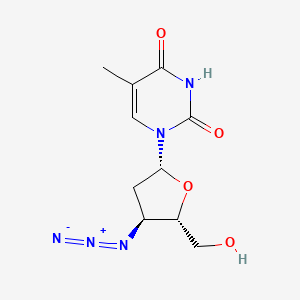 |
0.690 | ||
| ENC000944 | 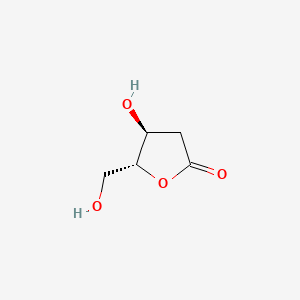 |
0.296 | D05RHI | 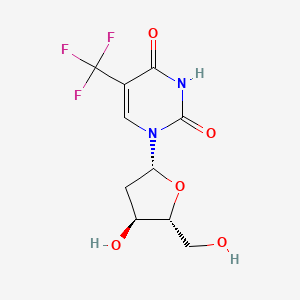 |
0.678 | ||
| ENC000063 | 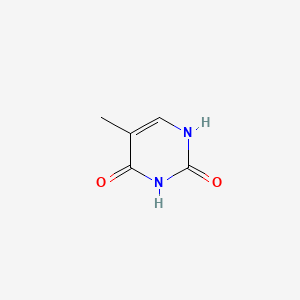 |
0.273 | D0Z8EX | 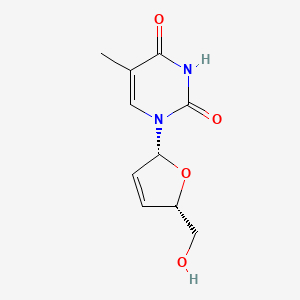 |
0.525 | ||
| ENC005481 | 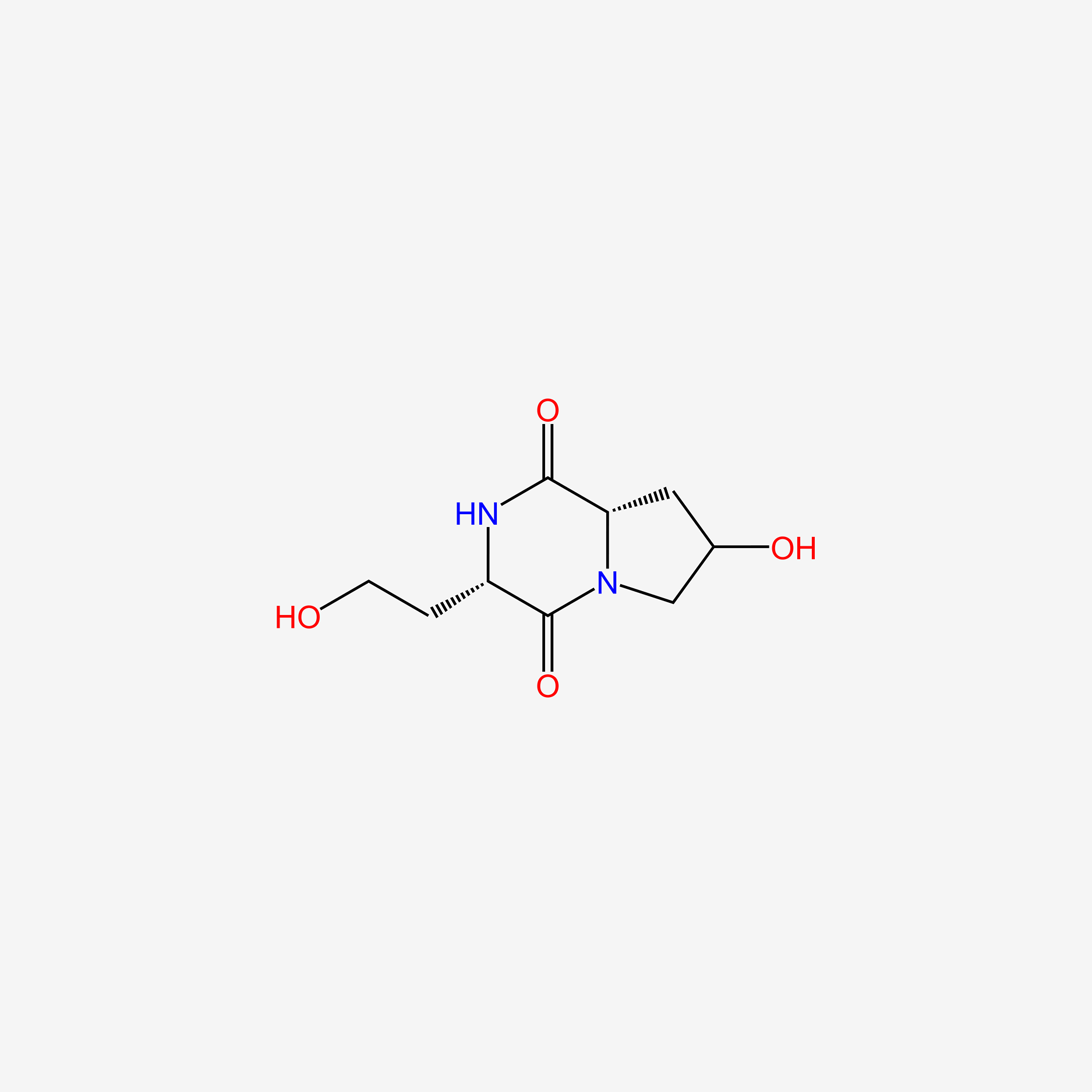 |
0.261 | D0X5XU | 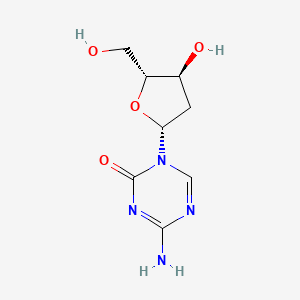 |
0.475 | ||
| ENC003178 | 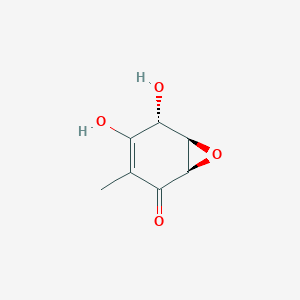 |
0.250 | D03TGJ | 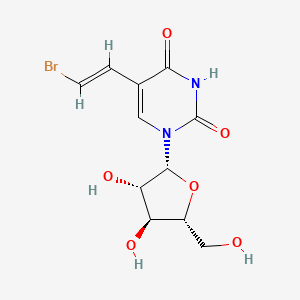 |
0.429 | ||
| ENC002632 | 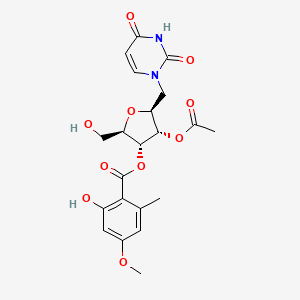 |
0.248 | D03KXY | 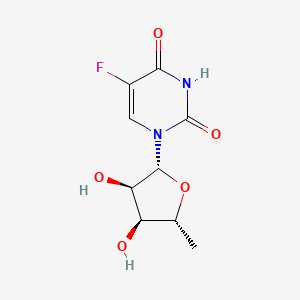 |
0.400 | ||