NPs Basic Information
|
Name |
DL-Pantolactone
|
| Molecular Formula | C6H10O3 | |
| IUPAC Name* |
3-hydroxy-4,4-dimethyloxolan-2-one
|
|
| SMILES |
CC1(COC(=O)C1O)C
|
|
| InChI |
InChI=1S/C6H10O3/c1-6(2)3-9-5(8)4(6)7/h4,7H,3H2,1-2H3
|
|
| InChIKey |
SERHXTVXHNVDKA-UHFFFAOYSA-N
|
|
| Synonyms |
DL-Pantolactone; 79-50-5; 3-hydroxy-4,4-dimethyldihydrofuran-2(3H)-one; pantoyl lactone; 3-hydroxy-4,4-dimethyloxolan-2-one; DL-Pantoyl Lactone; 2(3H)-Furanone, dihydro-3-hydroxy-4,4-dimethyl-; (+/-)-pantolactone; 52126-90-6; Dihydro-3-hydroxy-4,4-dimethyl-2(3H)-furanone; IID733HD2M; Pantolyl lactone; (+/-)-Dihydro-3-hydroxy-4,4-dimethyl-2(3H)-furanone; (D)-Pantolactone; (rs)-pantolactone; D-(-)-Pantolyl lactone; D-(-)-Pantoic acid lactone; UNII-IID733HD2M; 2(3H)-Furanone, dihydro-3-hydroxy-4,4-dimethyl-, (R)-; MFCD00005392; MFCD00216625; alpha-hydroxy-beta; D(-)Pantolactone; NSC 5926; EINECS 201-210-7; MFCD00064333; NSC 135788; AI3-23741; A-HYDROXY-,-DIMETHYL-G-BUTYROLACTONE; alpha-Hydroxy-beta,beta-dimethyl-gamma-butyrolactone; D(-)-2-Hydroxy-3,3-dimethyl-.gamma.-butyrolactone; DL-2-Hydroxy-3,3-dimethyl-gamma-butyrolactone; EC 201-210-7; Pantolactone DL-Form [MI]; 2(3H)-Furanone, dihydro-3-hydroxy-4,4-dimethyl-, D-(-)-; 3-Hydroxy-4,4-dimethyldihydro-2(3H)-furanone-, D-(-)- #; SCHEMBL152955; D-(-)-.alpha.Hydroxy-.beta.,.beta.-dimethyl-.gamma.-butyrolactone; PANTOLACTONE, (+-)-; (+-)-dihydro-3-hydroxy-4,4-dimethylfuran-2(3H)-one; CHEMBL4066633; (+/-)-PANTOYL LACTONE; DTXSID40861637; NSC5926; NSC8113; NSC8114; PANTOLACTONE, (+/-)-; AMY22249; NSC-5926; NSC-8113; NSC-8114; BBL005601; NSC135788; STK801814; AKOS003624024; Pro-vitamin B5, D-(-)-Pantolactone; HY-W053519; NSC-135788; SB37868; AS-12830; SY040562; SY110580; 2-hydroxy-3,3-dimethyl-gamma-butyrolactone; DB-053494; DB-056366; A8842; CS-0046126; FT-0624340; FT-0625501; FT-0635675; H-imidazo[1,2-a]pyridine-2-carboxylic acid; P0010; EN300-128349; 4,4-Dimethyl-3-hydroxy-dihydro-2(3H)-furanone; A832535; A839699; SR-01000944792; beta,beta-Dimethyl-alpha-hydroxy-gamma-butyrolactone; SR-01000944792-1; W-104261; (1)-Dihydro-3-hydroxy-4,4-dimethylfuran-2(3H)-one; 3-HYDROXYDIHYDRO-4,4-DIMETHYL-2(3H)-FURANONE; Q22829045; 2(3H)-Furanone, dihydro-3-hydroxy-4,4-dimethyl-, ( )-; (+/-)-2-HYDROXY-3,3-DIMETHYL-.GAMMA.-BUTYROLACTONE; 2(3H)-Furanone, dihydro-3-hydroxy-4,4-dimethyl-, (+/-)-; DL-alpha-Hydroxy-beta,beta-dimethyl-gamma-butyrolactone, 97%; 2(3H)-Furanone, dihydro-3-hydroxy-4,4-dimethyl-, (.+/-.)-; 3-Hydroxy-4,4-dimethyldihydrofuran-2(3H)-one (DL-Pantolactone); DL- alpha -Hydroxy- beta , beta -dimethyl- gamma -butyrolactone; BUTYRIC ACID, .ALPHA.,.GAMMA.-DIHYDROXY-.BETA.,.BETA.-DIMETHYL-, .GAMMA.-LACTONE; DL-alpha-Hydroxy-beta,beta-dimethyl-gamma-butyrolactone, purum, >=98.0% (T)
|
|
| CAS | 79-50-5 | |
| PubChem CID | 989 | |
| ChEMBL ID | CHEMBL4066633 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 130.14 | ALogp: | 0.5 |
| HBD: | 1 | HBA: | 3 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 46.5 | Aromatic Rings: | 1 |
| Heavy Atoms: | 9 | QED Weighted: | 0.481 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.507 | MDCK Permeability: | 0.00009070 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.009 |
| Human Intestinal Absorption (HIA): | 0.008 | 20% Bioavailability (F20%): | 0.005 |
| 30% Bioavailability (F30%): | 0.001 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.879 | Plasma Protein Binding (PPB): | 24.19% |
| Volume Distribution (VD): | 0.762 | Fu: | 84.87% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.06 | CYP1A2-substrate: | 0.135 |
| CYP2C19-inhibitor: | 0.033 | CYP2C19-substrate: | 0.818 |
| CYP2C9-inhibitor: | 0.014 | CYP2C9-substrate: | 0.476 |
| CYP2D6-inhibitor: | 0.007 | CYP2D6-substrate: | 0.608 |
| CYP3A4-inhibitor: | 0.006 | CYP3A4-substrate: | 0.185 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 6 | Half-life (T1/2): | 0.694 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.01 | Human Hepatotoxicity (H-HT): | 0.032 |
| Drug-inuced Liver Injury (DILI): | 0.097 | AMES Toxicity: | 0.122 |
| Rat Oral Acute Toxicity: | 0.039 | Maximum Recommended Daily Dose: | 0.057 |
| Skin Sensitization: | 0.293 | Carcinogencity: | 0.03 |
| Eye Corrosion: | 0.605 | Eye Irritation: | 0.956 |
| Respiratory Toxicity: | 0.356 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
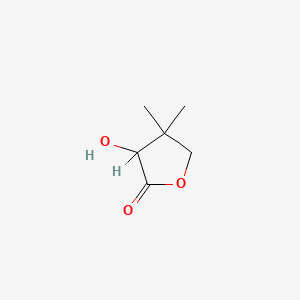
| ENC003753 | 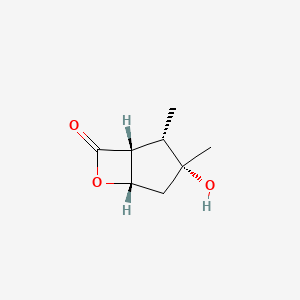 |
0.300 | D0H1QY |  |
0.238 | ||
| ENC004742 |  |
0.289 | D0U4VT |  |
0.231 | ||
| ENC004165 | 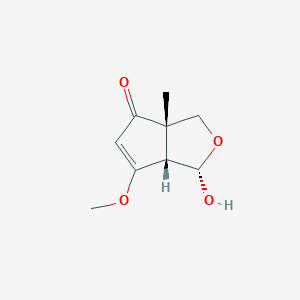 |
0.289 | D0Z8AA | 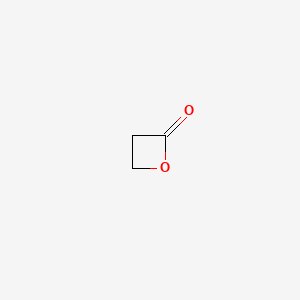 |
0.207 | ||
| ENC004166 | 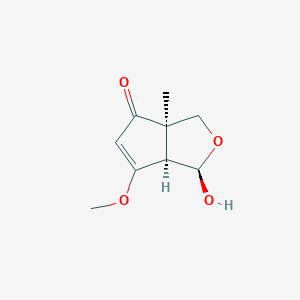 |
0.289 | D0U3GL | 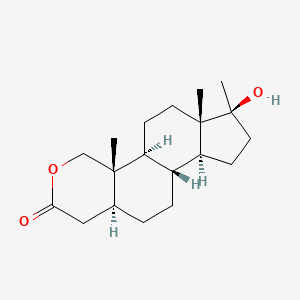 |
0.200 | ||
| ENC005579 | 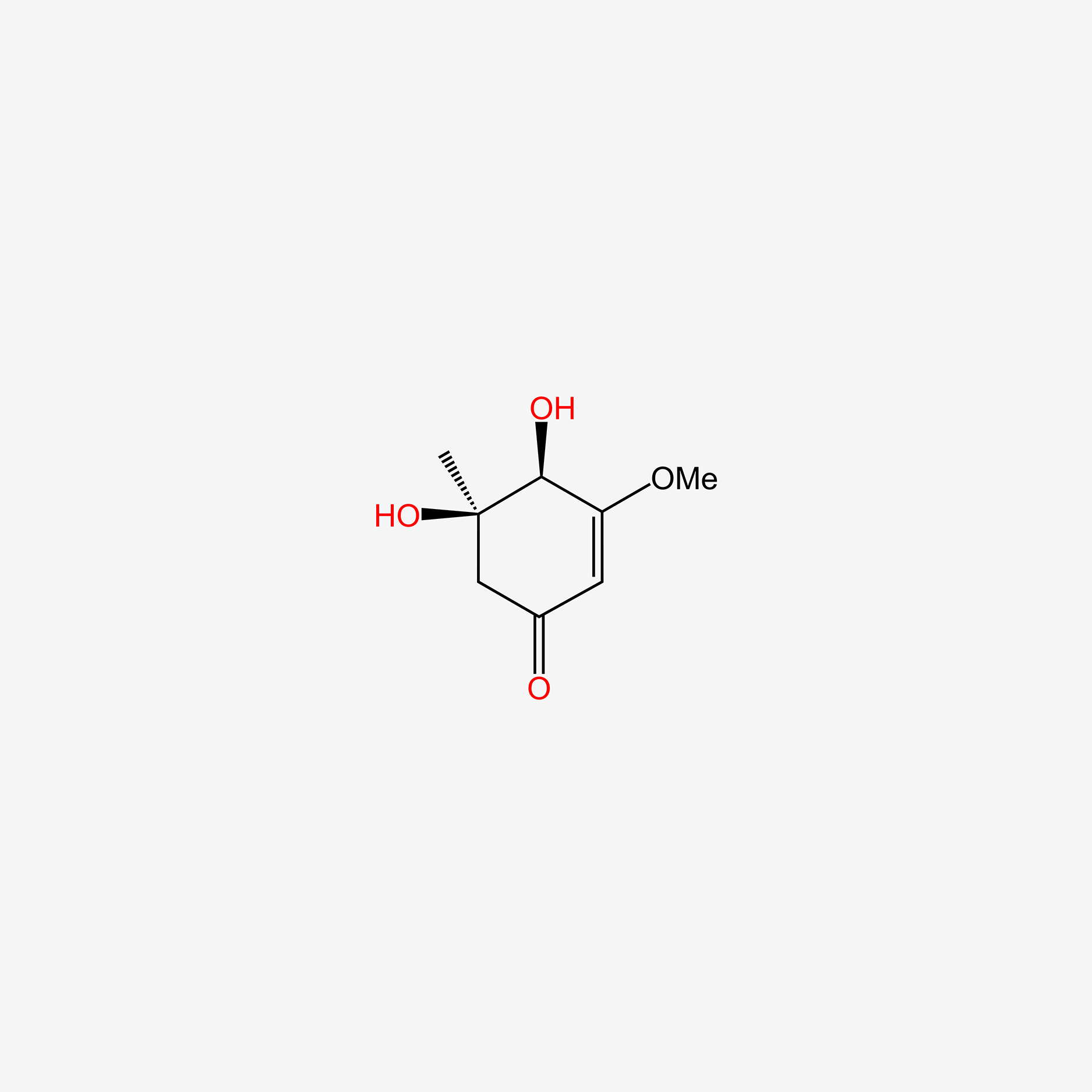 |
0.286 | D0G6AB | 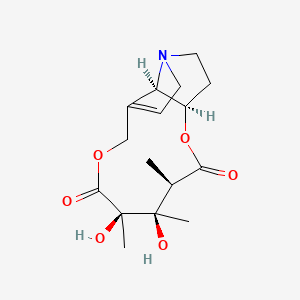 |
0.197 | ||
| ENC001525 | 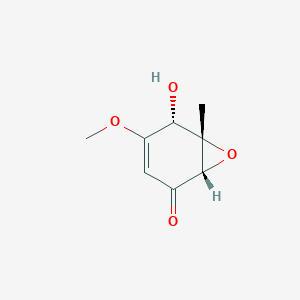 |
0.279 | D0Q4XQ | 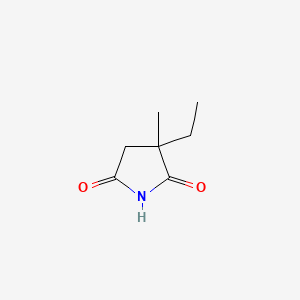 |
0.195 | ||
| ENC005472 | 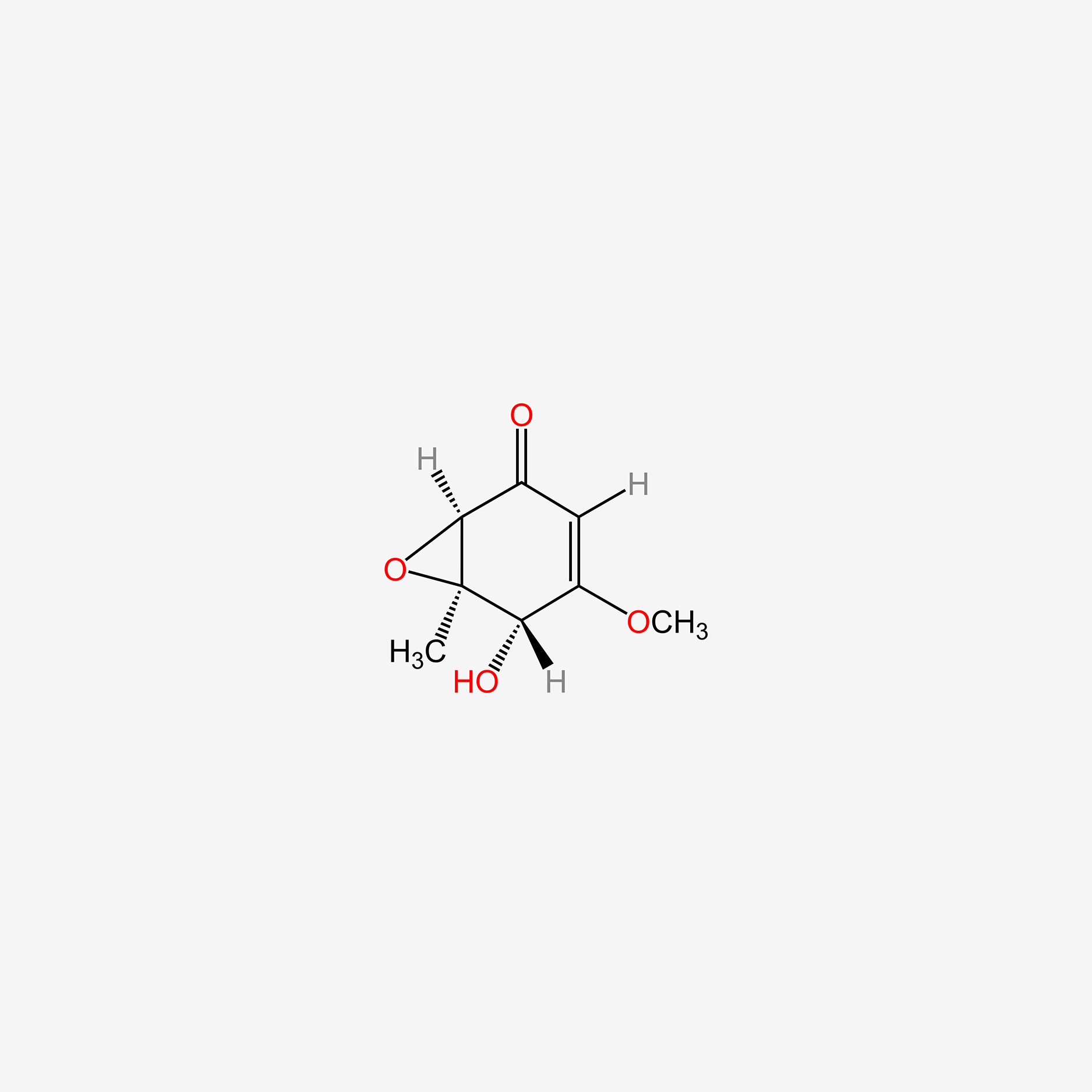 |
0.279 | D0H0BG | 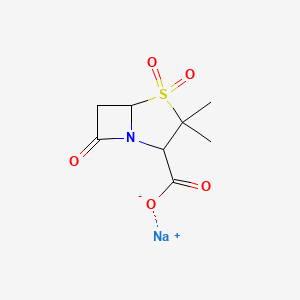 |
0.192 | ||
| ENC001789 | 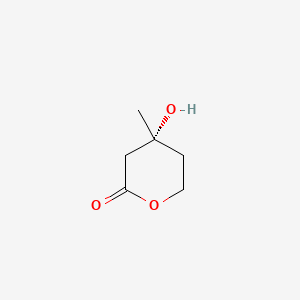 |
0.270 | D09JBP | 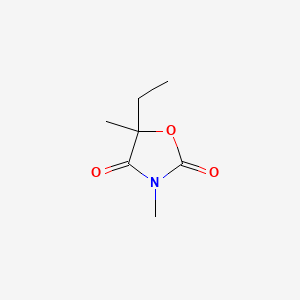 |
0.186 | ||
| ENC004863 | 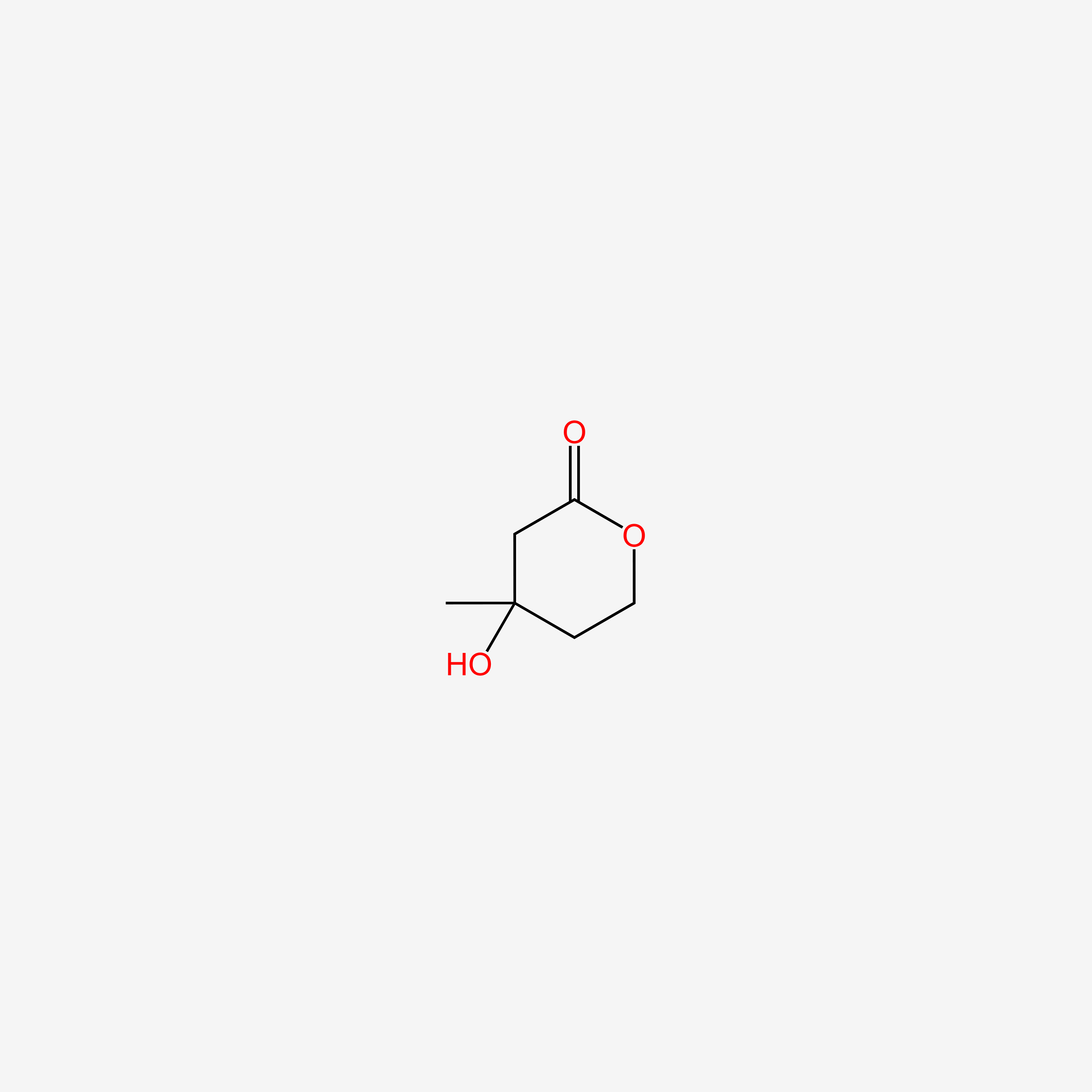 |
0.270 | D04VIS | 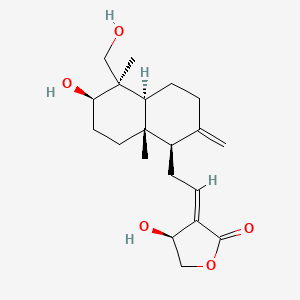 |
0.182 | ||
| ENC005897 | 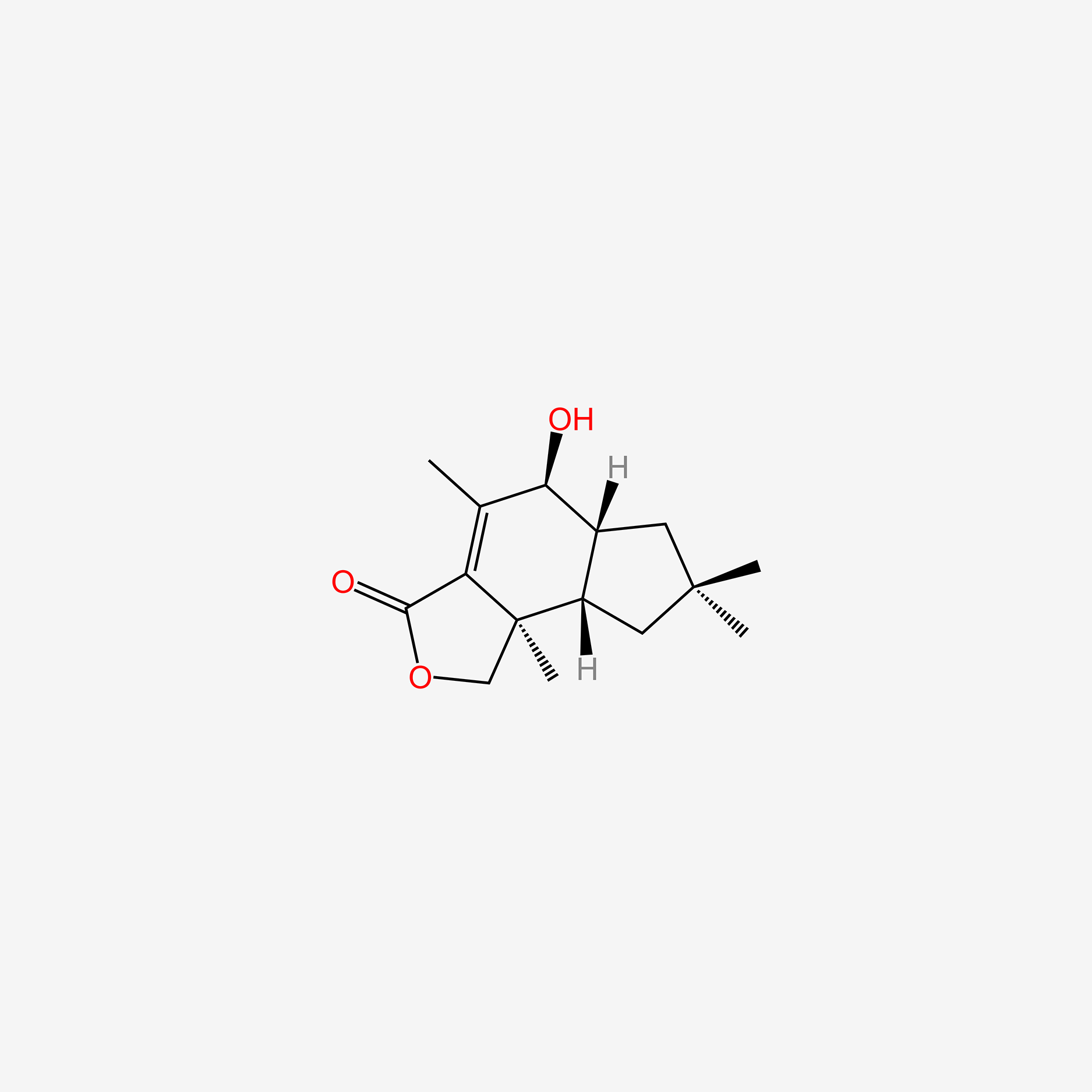 |
0.268 | D08BYK | 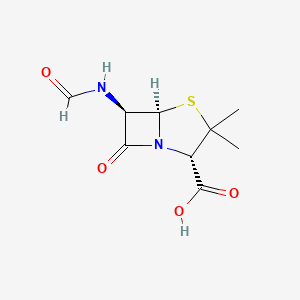 |
0.182 | ||