NPs Basic Information
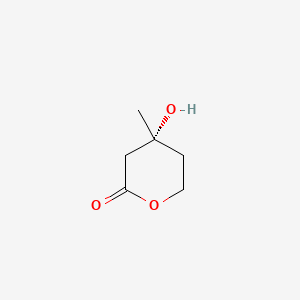
|
Name |
(R)-4-Hydroxy-4-methyltetrahydro-2H-pyran-2-one
|
| Molecular Formula | C6H10O3 | |
| IUPAC Name* |
(4R)-4-hydroxy-4-methyloxan-2-one
|
|
| SMILES |
C[C@]1(CCOC(=O)C1)O
|
|
| InChI |
InChI=1S/C6H10O3/c1-6(8)2-3-9-5(7)4-6/h8H,2-4H2,1H3/t6-/m1/s1
|
|
| InChIKey |
JYVXNLLUYHCIIH-ZCFIWIBFSA-N
|
|
| Synonyms |
19115-49-2; Mevalonolactone; (R)-4-Hydroxy-4-methyltetrahydro-2H-pyran-2-one; (-)-Mevalonolactone; (R)-(-)-Mevalonolactone; Adeka mevalonolactone; (3R)-Mevalonolactone; Mevalonolactone, (-)-; (-)-(R)-Mevalonic acid lactone; (-)-(R)-Mevalonolactone; 2H-Pyran-2-one, tetrahydro-4-hydroxy-4-methyl-, (4R)-; 2H-Pyran-2-one, tetrahydro-4-hydroxy-4-methyl-, (R)-; Mevalonic acid lactone; Mevalonic-D, L acid lactone; CHEBI:67849; 661X270Z3L; (R)-(-)-3-Hydroxy-3-methyl-5-pentanolide; D-Mevalonic Acid Lactone; r-mevalonolactone; UNII-661X270Z3L; D-Mevalonolactone; Prestwick_97; (R)-Mevalonolactone; R(-)Mevalonolactone; (4R)-4-hydroxy-4-methyloxan-2-one; Prestwick0_000750; Prestwick1_000750; Prestwick2_000750; R)-(-)-3-Hydroxy-3-methyl-5-pentanolide; SCHEMBL879; R-mevalonolactone, (-)-; D,L-mevalonic-acid-lactone; MEVALONOLACTONE [INCI]; SPBio_002679; CHEMBL1401520; HMS1570E22; ZINC4202723; MFCD01074894; AKOS017343730; SB47779; (R)-3-hydroxy-3-methyl-5-pentanolide; NCGC00016531-01; CAS-674-26-0; (R)-(-)-Mevalonolactone, >=90.0% (GC); M-6701; A937707; Q27136326
|
|
| CAS | 19115-49-2 | |
| PubChem CID | 6419891 | |
| ChEMBL ID | CHEMBL1401520 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 130.14 | ALogp: | -0.3 |
| HBD: | 1 | HBA: | 3 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 46.5 | Aromatic Rings: | 1 |
| Heavy Atoms: | 9 | QED Weighted: | 0.485 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.575 | MDCK Permeability: | 0.00006150 |
| Pgp-inhibitor: | 0.003 | Pgp-substrate: | 0.003 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.007 |
| 30% Bioavailability (F30%): | 0.061 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.871 | Plasma Protein Binding (PPB): | 12.10% |
| Volume Distribution (VD): | 0.671 | Fu: | 81.89% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.108 | CYP1A2-substrate: | 0.543 |
| CYP2C19-inhibitor: | 0.099 | CYP2C19-substrate: | 0.764 |
| CYP2C9-inhibitor: | 0.014 | CYP2C9-substrate: | 0.244 |
| CYP2D6-inhibitor: | 0.005 | CYP2D6-substrate: | 0.137 |
| CYP3A4-inhibitor: | 0.024 | CYP3A4-substrate: | 0.257 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.289 | Half-life (T1/2): | 0.797 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.024 | Human Hepatotoxicity (H-HT): | 0.033 |
| Drug-inuced Liver Injury (DILI): | 0.127 | AMES Toxicity: | 0.011 |
| Rat Oral Acute Toxicity: | 0.009 | Maximum Recommended Daily Dose: | 0.06 |
| Skin Sensitization: | 0.444 | Carcinogencity: | 0.064 |
| Eye Corrosion: | 0.961 | Eye Irritation: | 0.978 |
| Respiratory Toxicity: | 0.029 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC004863 | 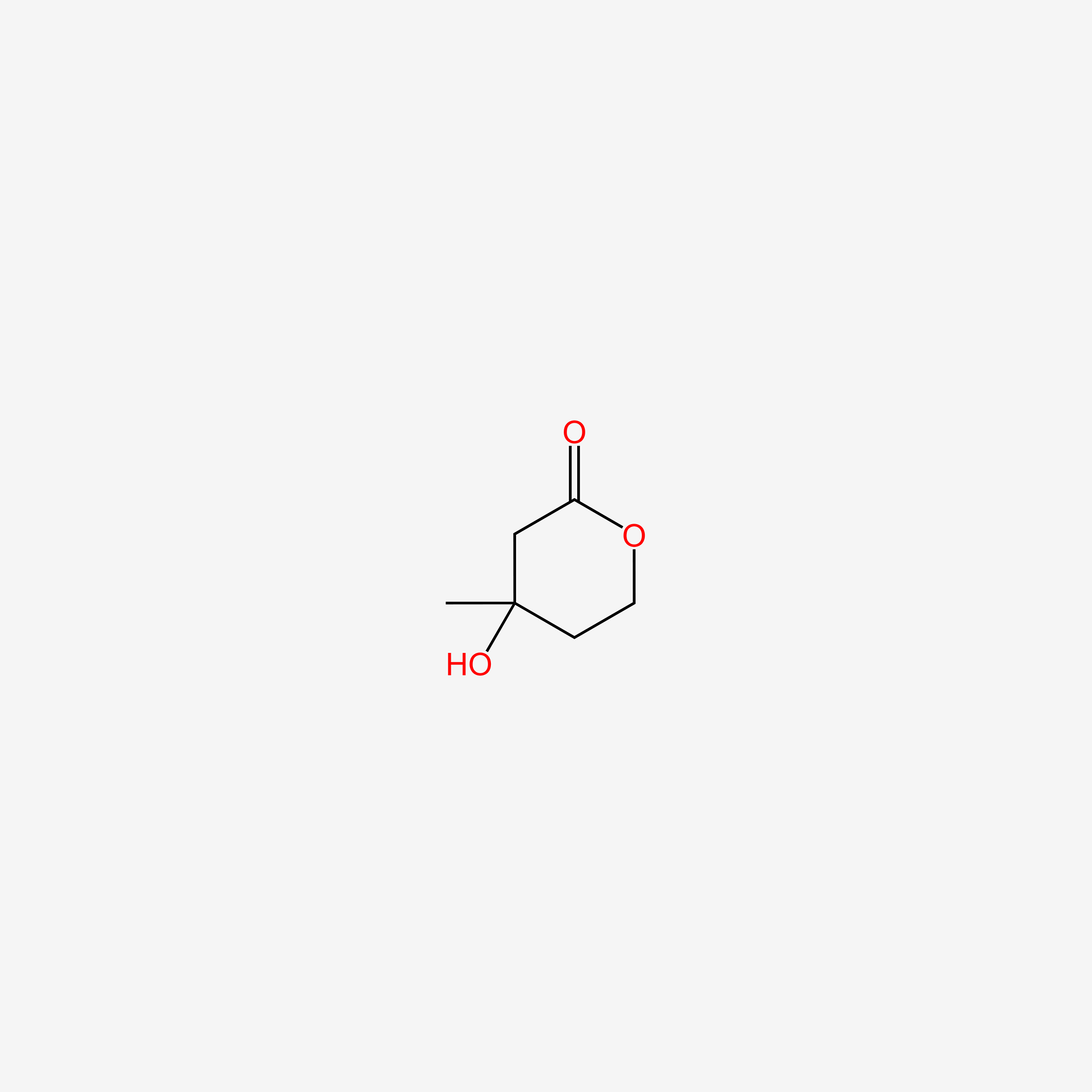 |
1.000 | D0Z8AA | 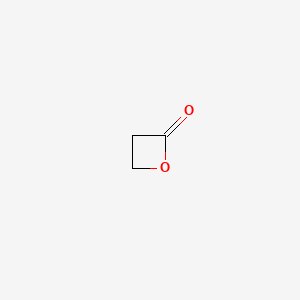 |
0.333 | ||
| ENC000184 | 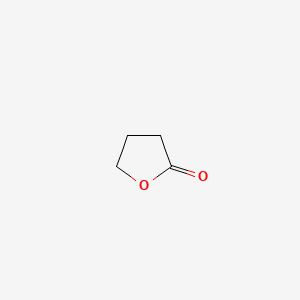 |
0.367 | D0H1QY |  |
0.233 | ||
| ENC003480 | 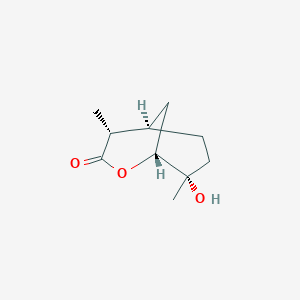 |
0.283 | D07QKN | 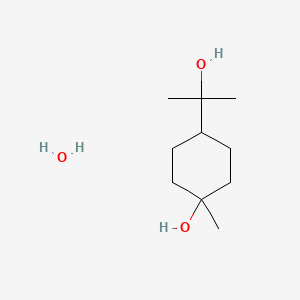 |
0.217 | ||
| ENC005088 | 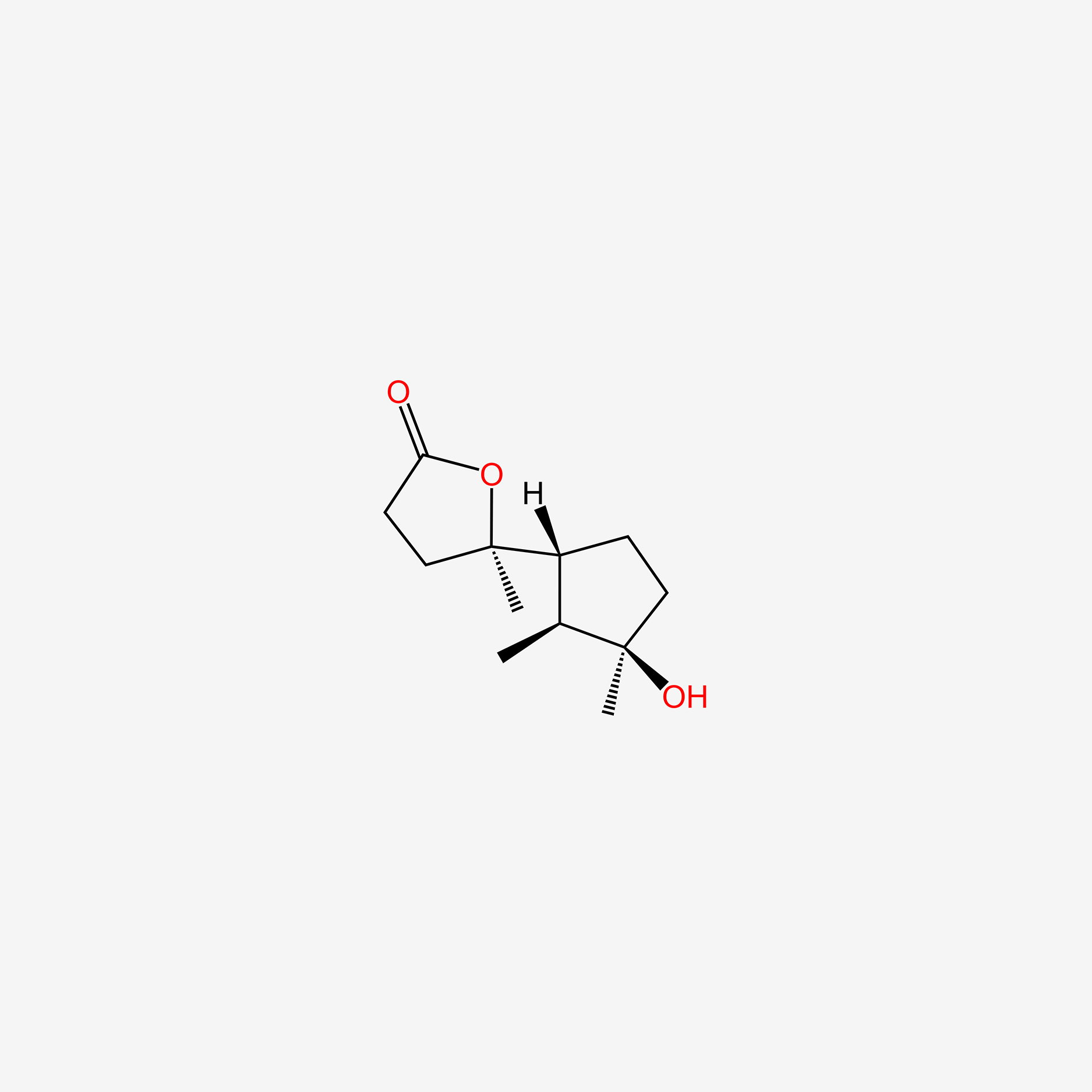 |
0.280 | D0U3GL | 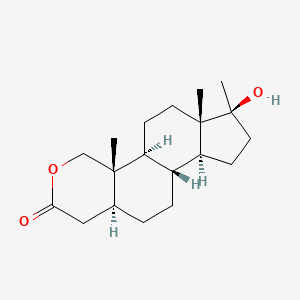 |
0.214 | ||
| ENC003670 |  |
0.273 | D0Q4XQ | 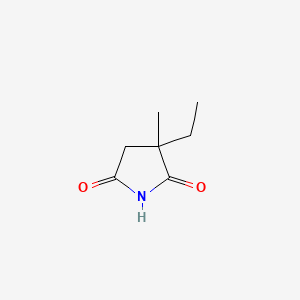 |
0.190 | ||
| ENC000051 | 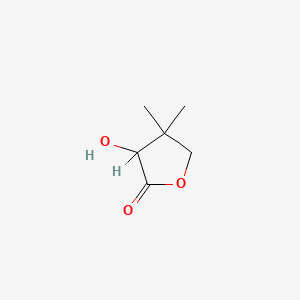 |
0.270 | D0G6AB | 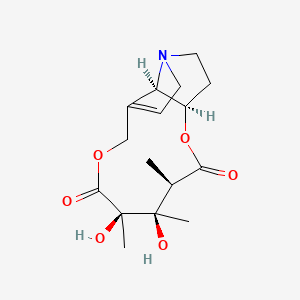 |
0.178 | ||
| ENC002170 | 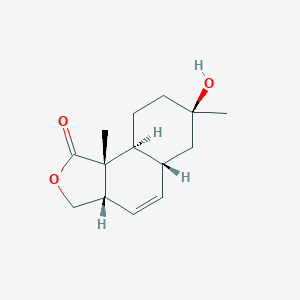 |
0.268 | D0A2AJ | 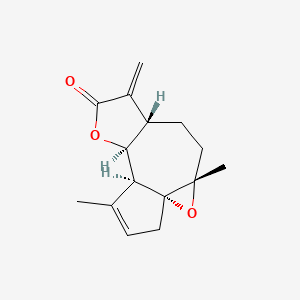 |
0.175 | ||
| ENC001341 | 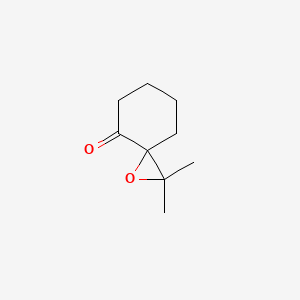 |
0.262 | D0Q6NZ | 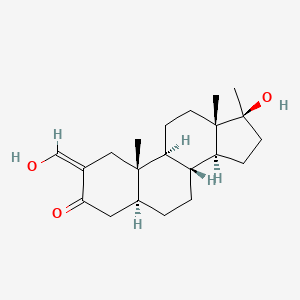 |
0.169 | ||
| ENC004767 | 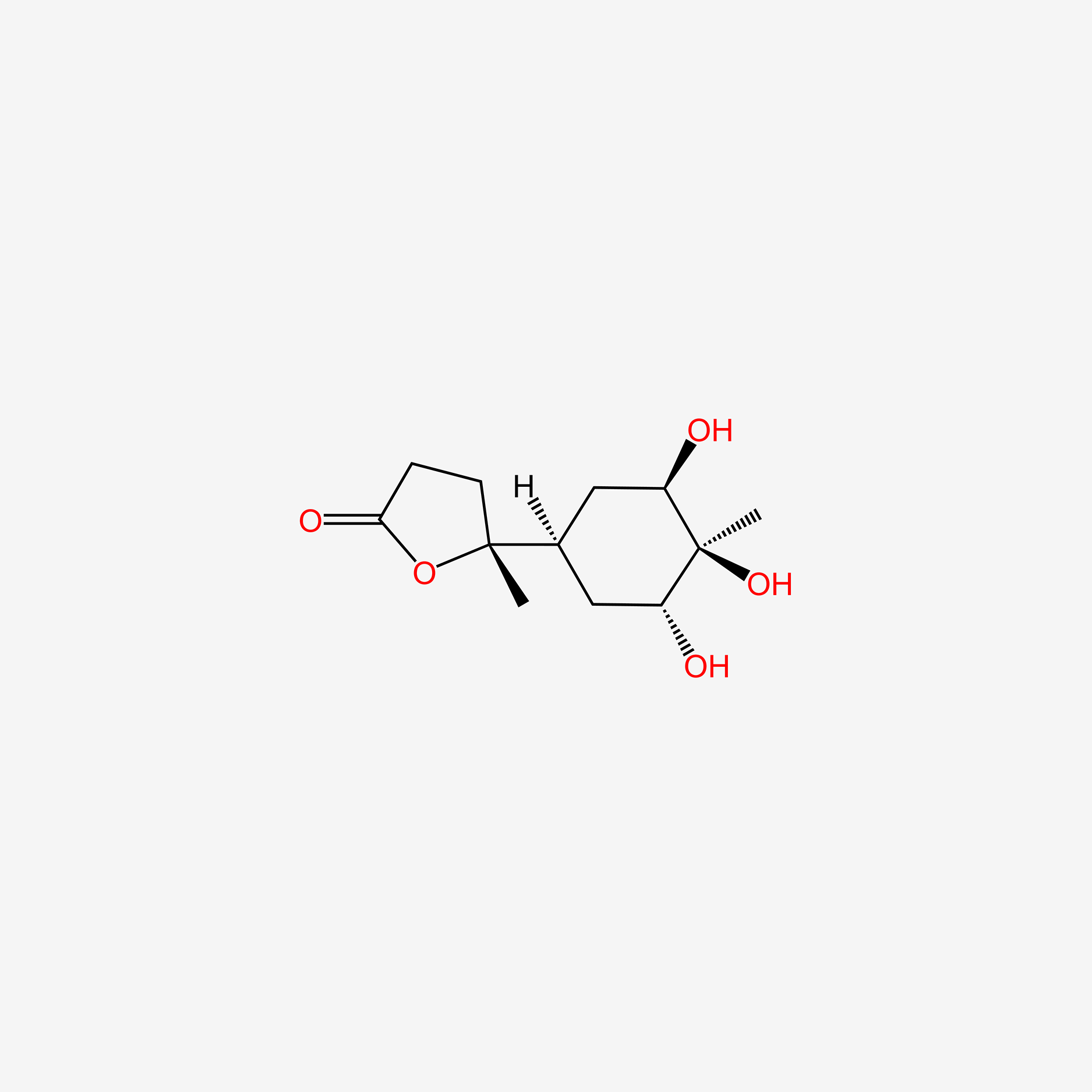 |
0.255 | D0U4VT | 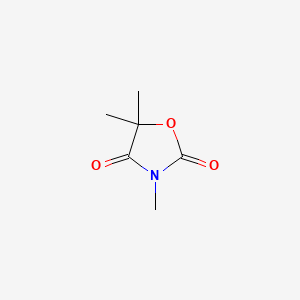 |
0.167 | ||
| ENC001047 | 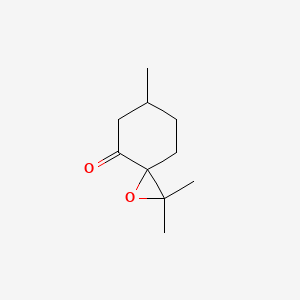 |
0.250 | D04VIS | 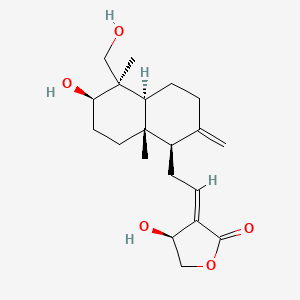 |
0.165 | ||