NPs Basic Information
|
Name |
Rhizopycnis acid B
|
| Molecular Formula | C13H16O5 | |
| IUPAC Name* |
3-[(3S)-3-carboxybutyl]-4-methoxybenzoic acid
|
|
| SMILES |
C[C@@H](CCC1=C(C=CC(=C1)C(=O)O)OC)C(=O)O
|
|
| InChI |
InChI=1S/C13H16O5/c1-8(12(14)15)3-4-9-7-10(13(16)17)5-6-11(9)18-2/h5-8H,3-4H2,1-2H3,(H,14,15)(H,16,17)/t8-/m0/s1
|
|
| InChIKey |
YANJIBCHXINHEA-QMMMGPOBSA-N
|
|
| Synonyms |
Rhizopycnis acid B
|
|
| CAS | NA | |
| PubChem CID | 146684112 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 252.26 | ALogp: | 2.1 |
| HBD: | 2 | HBA: | 5 |
| Rotatable Bonds: | 6 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 83.8 | Aromatic Rings: | 1 |
| Heavy Atoms: | 18 | QED Weighted: | 0.813 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.634 | MDCK Permeability: | 0.00002090 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.004 |
| Human Intestinal Absorption (HIA): | 0.031 | 20% Bioavailability (F20%): | 0.007 |
| 30% Bioavailability (F30%): | 0.59 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.219 | Plasma Protein Binding (PPB): | 89.66% |
| Volume Distribution (VD): | 0.199 | Fu: | 6.43% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.031 | CYP1A2-substrate: | 0.233 |
| CYP2C19-inhibitor: | 0.039 | CYP2C19-substrate: | 0.044 |
| CYP2C9-inhibitor: | 0.028 | CYP2C9-substrate: | 0.17 |
| CYP2D6-inhibitor: | 0.049 | CYP2D6-substrate: | 0.099 |
| CYP3A4-inhibitor: | 0.031 | CYP3A4-substrate: | 0.023 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 1.651 | Half-life (T1/2): | 0.94 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.02 | Human Hepatotoxicity (H-HT): | 0.265 |
| Drug-inuced Liver Injury (DILI): | 0.815 | AMES Toxicity: | 0.004 |
| Rat Oral Acute Toxicity: | 0.049 | Maximum Recommended Daily Dose: | 0.009 |
| Skin Sensitization: | 0.069 | Carcinogencity: | 0.022 |
| Eye Corrosion: | 0.018 | Eye Irritation: | 0.806 |
| Respiratory Toxicity: | 0.029 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
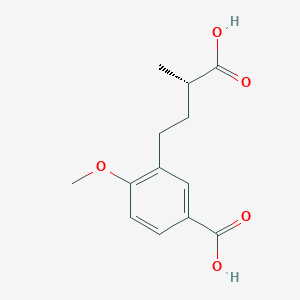
| ENC004157 | 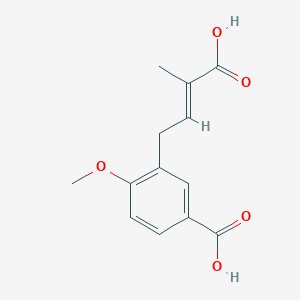 |
0.600 | D02XJY | 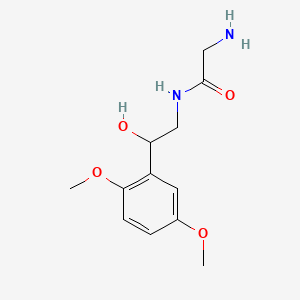 |
0.329 | ||
| ENC000296 |  |
0.481 | D0DJ1B |  |
0.301 | ||
| ENC001090 | 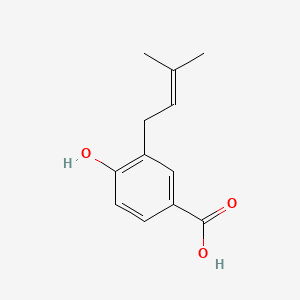 |
0.419 | D0U9QU | 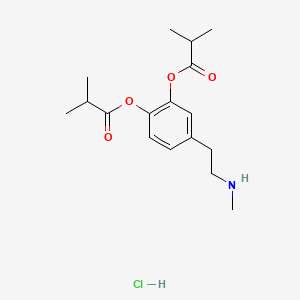 |
0.301 | ||
| ENC004987 | 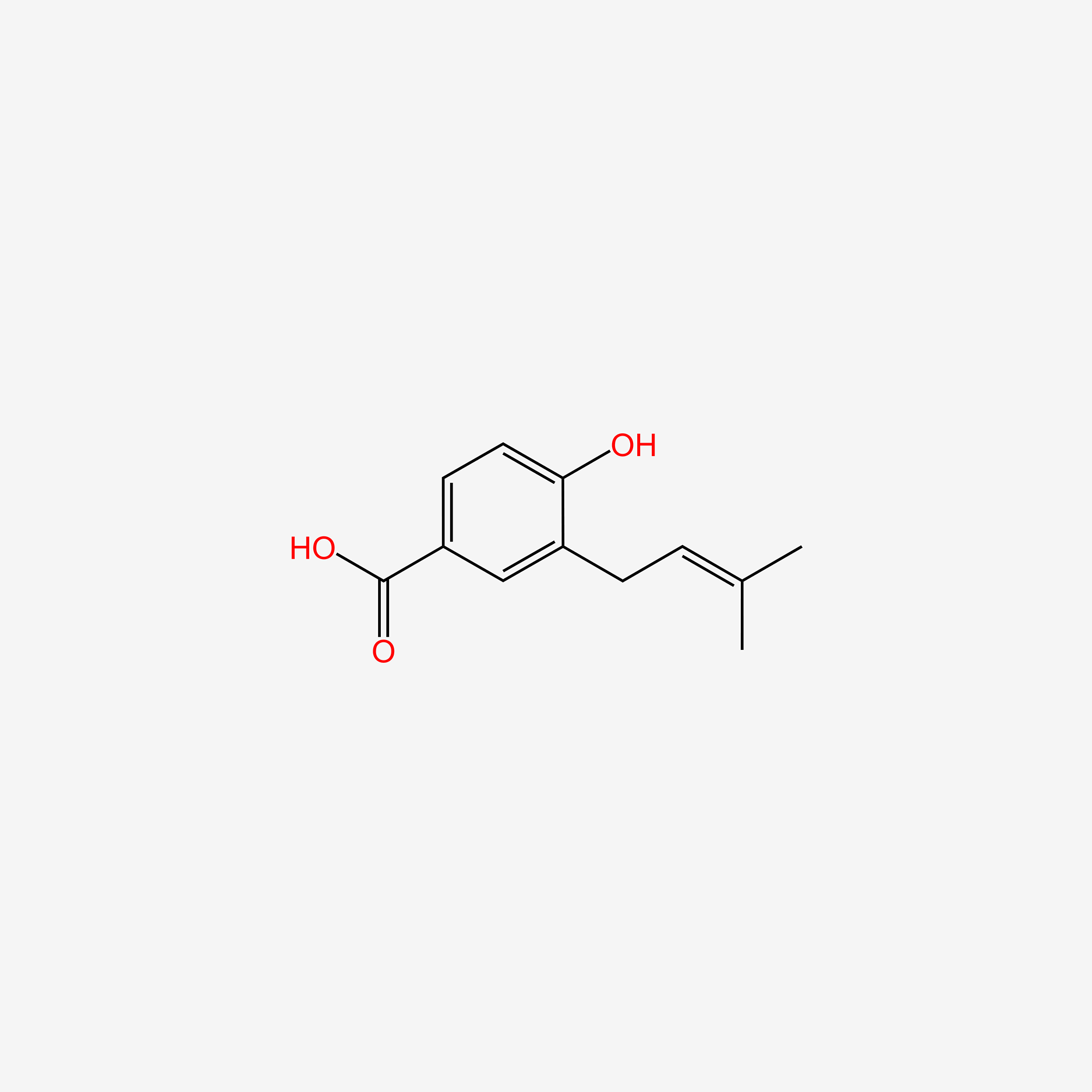 |
0.419 | D0E9CD |  |
0.300 | ||
| ENC000764 | 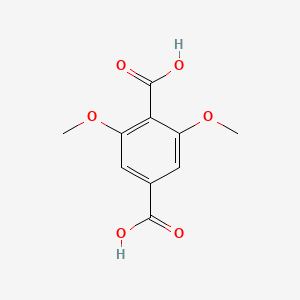 |
0.406 | D0GY5Z | 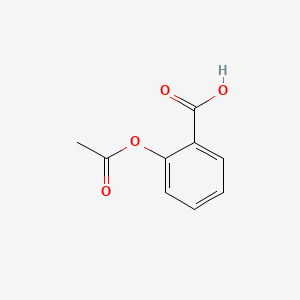 |
0.297 | ||
| ENC004195 | 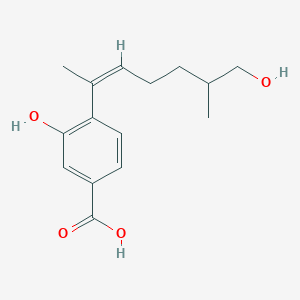 |
0.394 | D09GYT | 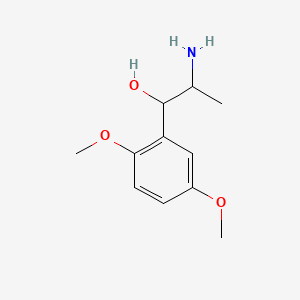 |
0.294 | ||
| ENC004196 |  |
0.394 | D0E6OC | 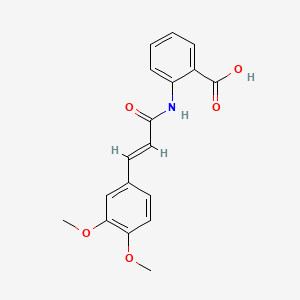 |
0.292 | ||
| ENC005619 | 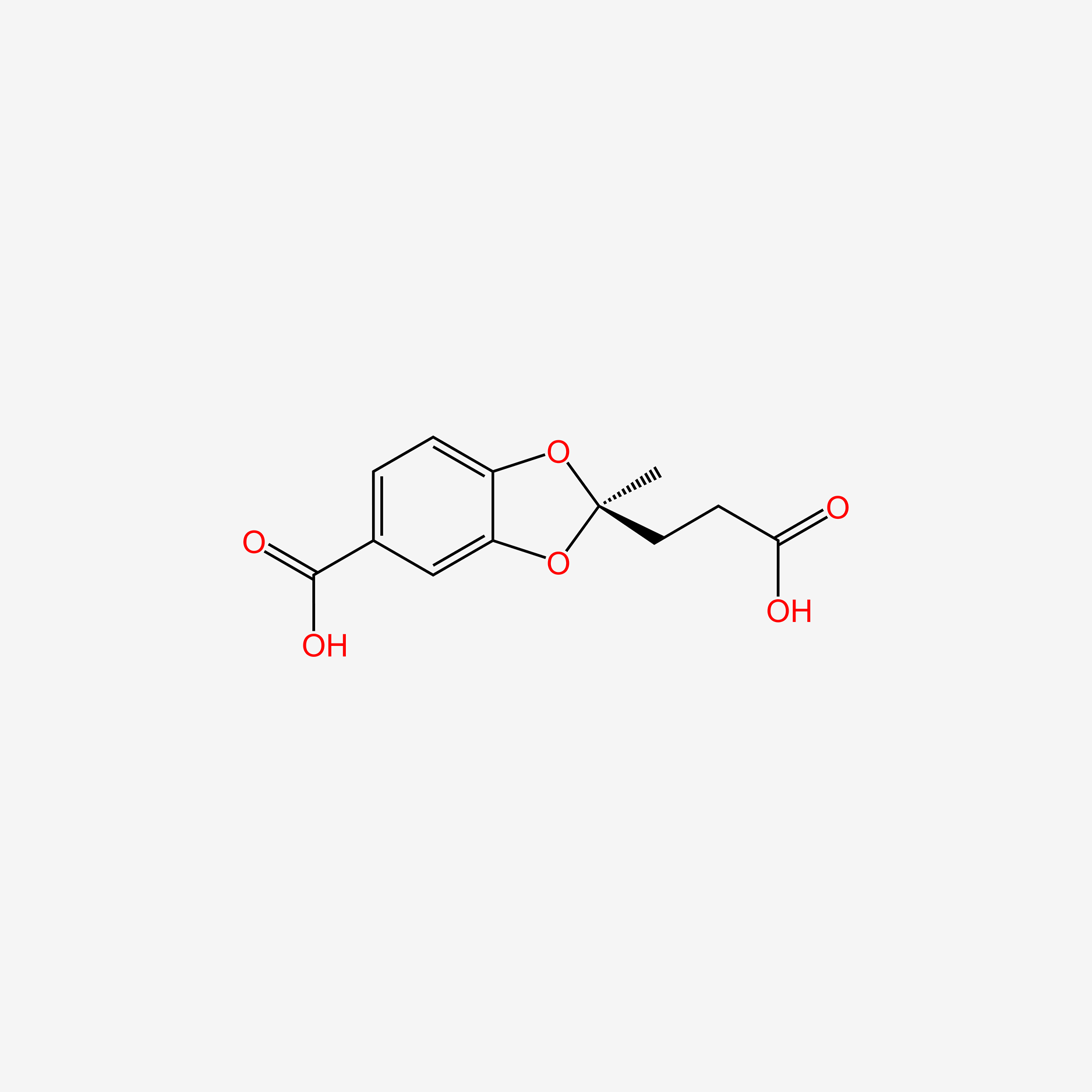 |
0.386 | D07BPS | 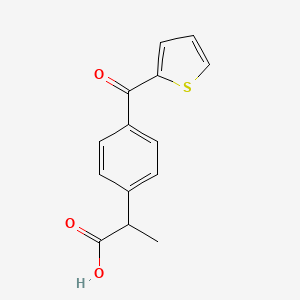 |
0.289 | ||
| ENC004350 | 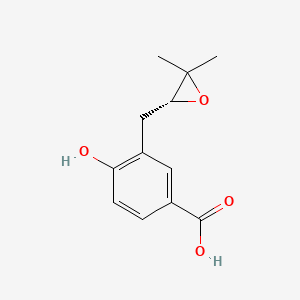 |
0.385 | D0HD9K | 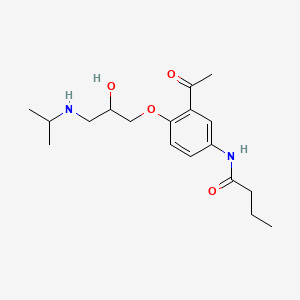 |
0.284 | ||
| ENC000777 | 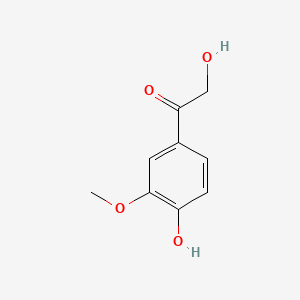 |
0.383 | D03LGG | 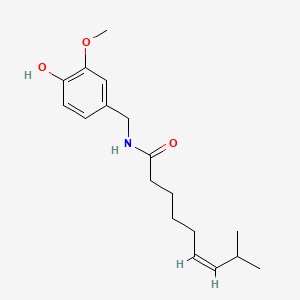 |
0.282 | ||