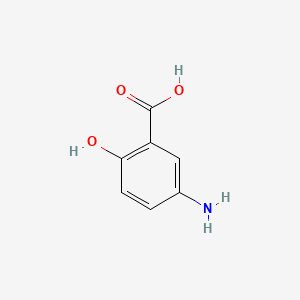
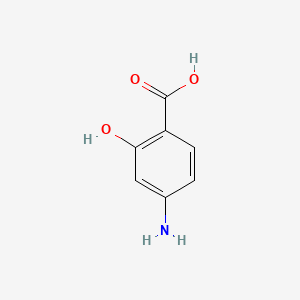
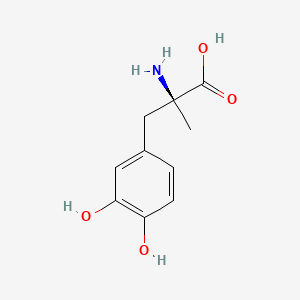
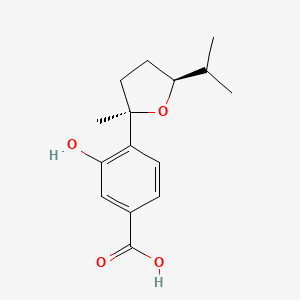
NPs Basic Information

|
Name |
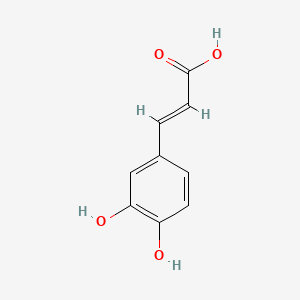
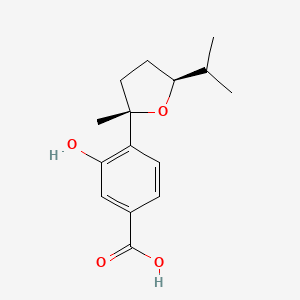
Terreprenphenol B
|
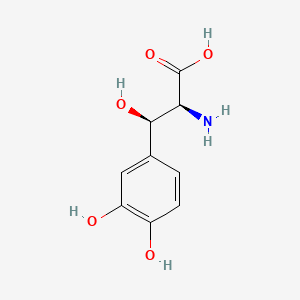
| Molecular Formula | C12H14O4 | |
| IUPAC Name* |
3-[[(2R)-3,3-dimethyloxiran-2-yl]methyl]-4-hydroxybenzoic acid
|
|
| SMILES |
CC1([C@H](O1)CC2=C(C=CC(=C2)C(=O)O)O)C
|
|
| InChI |
InChI=1S/C12H14O4/c1-12(2)10(16-12)6-8-5-7(11(14)15)3-4-9(8)13/h3-5,10,13H,6H2,1-2H3,(H,14,15)/t10-/m1/s1
|
|
| InChIKey |
FTOBXGMWCGITQF-SNVBAGLBSA-N
|
|
| Synonyms |
Terreprenphenol B
|
|
| CAS | NA | |
| PubChem CID | 156582149 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Physi-Chem Properties
| Molecular Weight: | 222.24 | ALogp: | 1.7 |
| HBD: | 2 | HBA: | 4 |
| Rotatable Bonds: | 3 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 70.1 | Aromatic Rings: | 2 |
| Heavy Atoms: | 16 | QED Weighted: | 0.77 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.206 | MDCK Permeability: | 0.00001040 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.008 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.007 |
| 30% Bioavailability (F30%): | 0.226 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.147 | Plasma Protein Binding (PPB): | 58.75% |
| Volume Distribution (VD): | 0.238 | Fu: | 24.86% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.036 | CYP1A2-substrate: | 0.092 |
| CYP2C19-inhibitor: | 0.028 | CYP2C19-substrate: | 0.054 |
| CYP2C9-inhibitor: | 0.071 | CYP2C9-substrate: | 0.161 |
| CYP2D6-inhibitor: | 0.025 | CYP2D6-substrate: | 0.114 |
| CYP3A4-inhibitor: | 0.034 | CYP3A4-substrate: | 0.117 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 4.584 | Half-life (T1/2): | 0.912 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.027 | Human Hepatotoxicity (H-HT): | 0.42 |
| Drug-inuced Liver Injury (DILI): | 0.949 | AMES Toxicity: | 0.219 |
| Rat Oral Acute Toxicity: | 0.505 | Maximum Recommended Daily Dose: | 0.382 |
| Skin Sensitization: | 0.424 | Carcinogencity: | 0.529 |
| Eye Corrosion: | 0.032 | Eye Irritation: | 0.874 |
| Respiratory Toxicity: | 0.223 |