NPs Basic Information
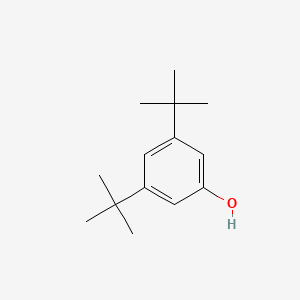
|
Name |
3,5-Di-tert-butylphenol
|
| Molecular Formula | C14H22O | |
| IUPAC Name* |
3,5-ditert-butylphenol
|
|
| SMILES |
CC(C)(C)C1=CC(=CC(=C1)O)C(C)(C)C
|
|
| InChI |
InChI=1S/C14H22O/c1-13(2,3)10-7-11(14(4,5)6)9-12(15)8-10/h7-9,15H,1-6H3
|
|
| InChIKey |
ZDWSNKPLZUXBPE-UHFFFAOYSA-N
|
|
| Synonyms |
3,5-Di-tert-butylphenol; 1138-52-9; 3,5-ditert-butylphenol; 3,5-Di-t-butylphenol; Phenol, 3,5-bis(1,1-dimethylethyl)-; Phenol, 3,5-di-tert-butyl-; C14H22O; 3,5-di-tert-butyl phenol; F4SMH8G9W7; CHEMBL321841; MFCD00008829; NSC-68209; 3,5-BIS(1,1-DIMETHYLETHYL)PHENOL; EINECS 214-513-4; NSC 68209; UNII-F4SMH8G9W7; 3,5-bis(tert-butyl)phenol; NSC68209; 3,5-di-tertbutylphenol; ChemDiv3_014149; 3,5-di(tert-butyl)phenol; 3,5-di-tertiary butylphenol; SCHEMBL18268; Phenol, 3,5-bis(t-butyl); DTXSID3061558; ZDWSNKPLZUXBPE-UHFFFAOYSA-; HMS1513D03; ACT09690; BAA13852; ZINC1081265; 3,5-bis(1,1-dimethylethyl)-pheno; BDBM50409534; AKOS001387910; AM82867; CCG-202926; CS-W021820; HY-W041080; IDI1_029947; NCGC00337070-01; SY047068; DB-006313; D5978; FT-0614739; EN300-20888; O10925; AB01330491-02; AG-672/25002600; SR-01000597208; J-511377; SR-01000597208-1; Q27277633; 3,5-Di-tert-butylphenol 100 microg/mL in Acetonitrile; Z104484466; N,O-Diethyl-N-(4-phenyl-4-(pyridin-2-yl)butyl)hydroxylamine
|
|
| CAS | 1138-52-9 | |
| PubChem CID | 70825 | |
| ChEMBL ID | CHEMBL321841 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 206.32 | ALogp: | 4.9 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 20.2 | Aromatic Rings: | 1 |
| Heavy Atoms: | 15 | QED Weighted: | 0.656 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.107 | MDCK Permeability: | 0.00000798 |
| Pgp-inhibitor: | 0.158 | Pgp-substrate: | 0.008 |
| Human Intestinal Absorption (HIA): | 0.546 | 20% Bioavailability (F20%): | 0.998 |
| 30% Bioavailability (F30%): | 0.994 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.578 | Plasma Protein Binding (PPB): | 96.63% |
| Volume Distribution (VD): | 4.514 | Fu: | 6.81% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.944 | CYP1A2-substrate: | 0.886 |
| CYP2C19-inhibitor: | 0.86 | CYP2C19-substrate: | 0.709 |
| CYP2C9-inhibitor: | 0.701 | CYP2C9-substrate: | 0.859 |
| CYP2D6-inhibitor: | 0.909 | CYP2D6-substrate: | 0.663 |
| CYP3A4-inhibitor: | 0.567 | CYP3A4-substrate: | 0.527 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.521 | Half-life (T1/2): | 0.348 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.031 | Human Hepatotoxicity (H-HT): | 0.089 |
| Drug-inuced Liver Injury (DILI): | 0.037 | AMES Toxicity: | 0.002 |
| Rat Oral Acute Toxicity: | 0.08 | Maximum Recommended Daily Dose: | 0.908 |
| Skin Sensitization: | 0.754 | Carcinogencity: | 0.024 |
| Eye Corrosion: | 0.967 | Eye Irritation: | 0.983 |
| Respiratory Toxicity: | 0.522 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000898 | 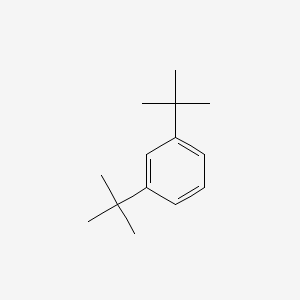 |
0.574 | D0M8RC | 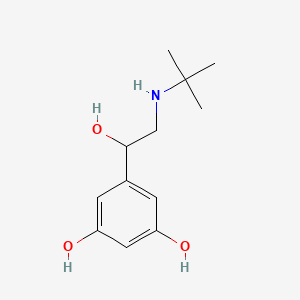 |
0.333 | ||
| ENC000185 | 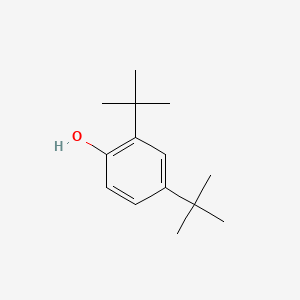 |
0.551 | D0Y4DY | 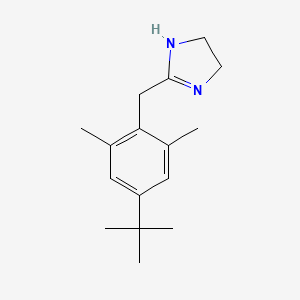 |
0.299 | ||
| ENC000744 | 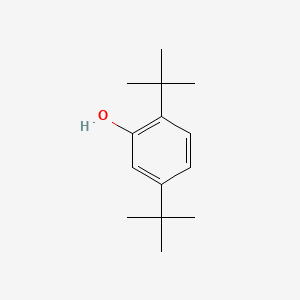 |
0.551 | D00NJL | 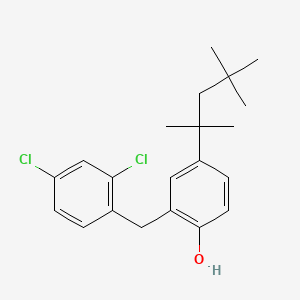 |
0.291 | ||
| ENC005113 | 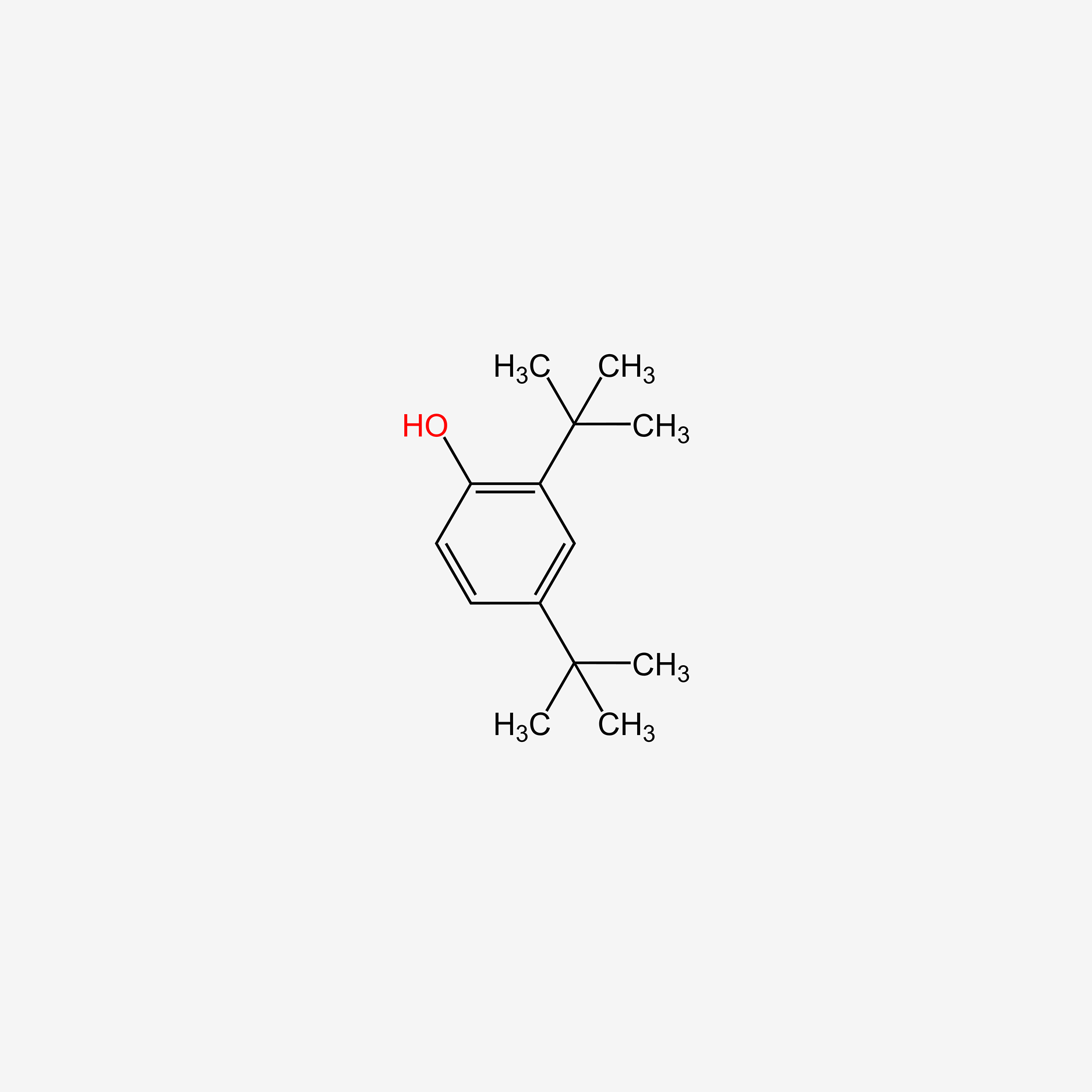 |
0.551 | D0W0BF |  |
0.289 | ||
| ENC000725 | 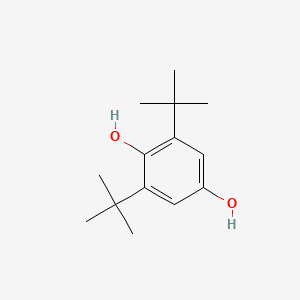 |
0.529 | D0W7WC | 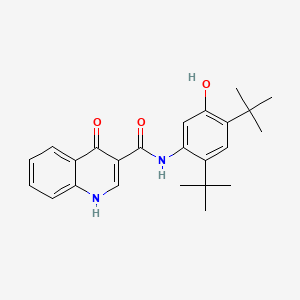 |
0.289 | ||
| ENC001398 | 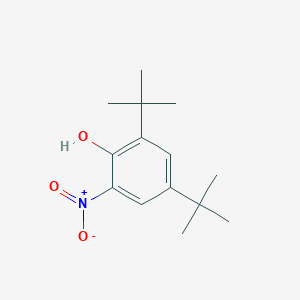 |
0.509 | D06YPU | 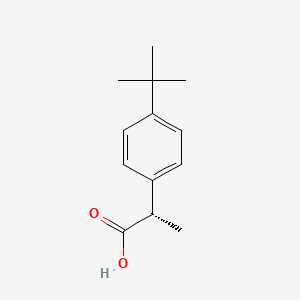 |
0.283 | ||
| ENC000346 | 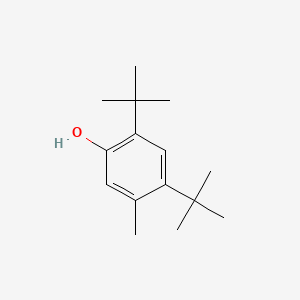 |
0.472 | D01JFT | 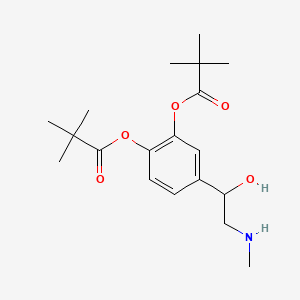 |
0.272 | ||
| ENC000079 | 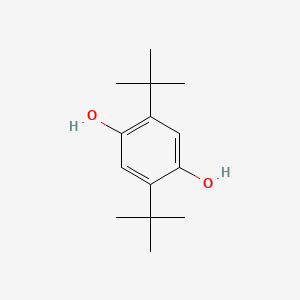 |
0.472 | D0H2DQ | 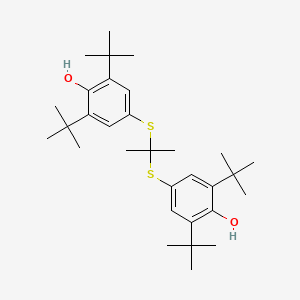 |
0.270 | ||
| ENC000610 |  |
0.472 | D07EXH | 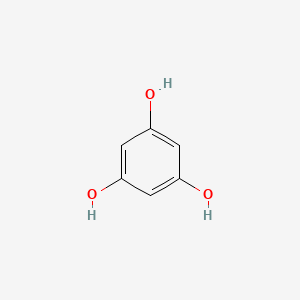 |
0.265 | ||
| ENC000611 | 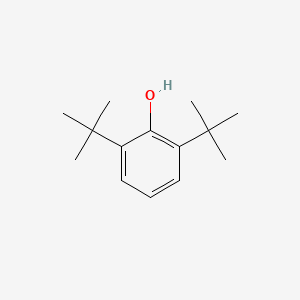 |
0.462 | D0K5CB | 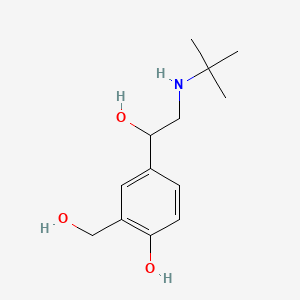 |
0.239 | ||