NPs Basic Information
|
Name |
2,6-Di-tert-butylhydroquinone
|
| Molecular Formula | C14H22O2 | |
| IUPAC Name* |
2,6-ditert-butylbenzene-1,4-diol
|
|
| SMILES |
CC(C)(C)C1=CC(=CC(=C1O)C(C)(C)C)O
|
|
| InChI |
InChI=1S/C14H22O2/c1-13(2,3)10-7-9(15)8-11(12(10)16)14(4,5)6/h7-8,15-16H,1-6H3
|
|
| InChIKey |
JFGVTUJBHHZRAB-UHFFFAOYSA-N
|
|
| Synonyms |
2,6-Di-tert-butylhydroquinone; 2444-28-2; 2,6-di-tert-butylbenzene-1,4-diol; 1,4-Benzenediol, 2,6-bis(1,1-dimethylethyl)-; 2,6-ditert-butylbenzene-1,4-diol; 2,6-Di-tert-butyl-1,4-benzenediol; 2,6-Di-tert-butyl-hydroquinone; Hydroquinone, 2,6-di-tert-butyl-; RW7RBM89DC; 2,6-di-t-butylhydroquinone; 2,6-Di-tert-butyl-1,4-dihydroxybenzene; UNII-RW7RBM89DC; EINECS 219-481-5; 2,5-ditertbutylhydroquinone; Oprea1_865590; SCHEMBL39962; 2,6-di(t-butyl)hydroquinone; 3,5-Di-tert-butylhydroquinone; CHEMBL375695; GTPL5486; 2,6-di tert.butyl hydroquinone; 2,6-di-tert.butyl-hydroquinone; DTXSID5062423; CHEBI:174144; CAA44428; ZINC1841214; MFCD00458424; STK365476; AKOS005442455; 2,6-Ditert-butyl-1,4-benzenediol #; 2,6-DI-TERT-BUTYL-P-HYDROQUINONE; SY264943; 2,6-DI-TERT-BUTYLBENZOHYDROQUINONE; CS-0260246; 2,6-DI-TERT-BUTYL-1,4-HYDROQUINONE; 3,5-DI-TERT-BUTYL-1,4-HYDROQUINONE; 2,6-Bis(1,1-dimethylethyl)-1,4-Benzenediol; EN300-7472373; 2,6-Bis(1,1-dimethylethyl)-1,4-benzenediol, 9CI; Q27075212
|
|
| CAS | 2444-28-2 | |
| PubChem CID | 75550 | |
| ChEMBL ID | CHEMBL375695 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 222.32 | ALogp: | 4.6 |
| HBD: | 2 | HBA: | 2 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 40.5 | Aromatic Rings: | 1 |
| Heavy Atoms: | 16 | QED Weighted: | 0.639 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.004 | MDCK Permeability: | 0.00001280 |
| Pgp-inhibitor: | 0.606 | Pgp-substrate: | 0.009 |
| Human Intestinal Absorption (HIA): | 0.696 | 20% Bioavailability (F20%): | 0.998 |
| 30% Bioavailability (F30%): | 0.993 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.334 | Plasma Protein Binding (PPB): | 97.41% |
| Volume Distribution (VD): | 4.385 | Fu: | 5.44% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.896 | CYP1A2-substrate: | 0.913 |
| CYP2C19-inhibitor: | 0.479 | CYP2C19-substrate: | 0.53 |
| CYP2C9-inhibitor: | 0.494 | CYP2C9-substrate: | 0.916 |
| CYP2D6-inhibitor: | 0.874 | CYP2D6-substrate: | 0.884 |
| CYP3A4-inhibitor: | 0.341 | CYP3A4-substrate: | 0.449 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 8.294 | Half-life (T1/2): | 0.766 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.011 | Human Hepatotoxicity (H-HT): | 0.045 |
| Drug-inuced Liver Injury (DILI): | 0.018 | AMES Toxicity: | 0.008 |
| Rat Oral Acute Toxicity: | 0.13 | Maximum Recommended Daily Dose: | 0.845 |
| Skin Sensitization: | 0.919 | Carcinogencity: | 0.031 |
| Eye Corrosion: | 0.904 | Eye Irritation: | 0.954 |
| Respiratory Toxicity: | 0.751 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
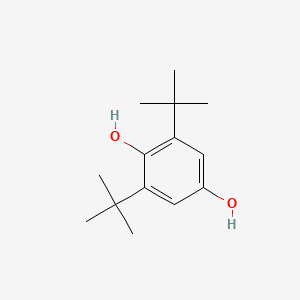
| ENC000610 |  |
0.739 | D0H2DQ | 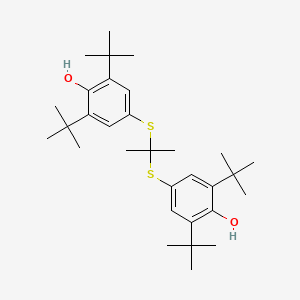 |
0.387 | ||
| ENC000708 | 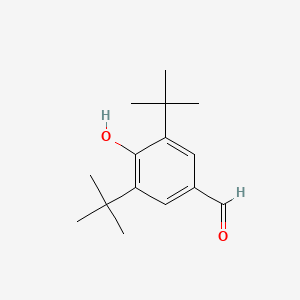 |
0.694 | D0W7WC | 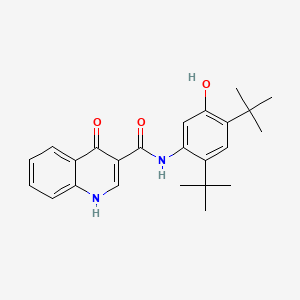 |
0.341 | ||
| ENC000079 |  |
0.667 | D0M8RC |  |
0.323 | ||
| ENC000346 |  |
0.600 | D09EBS |  |
0.319 | ||
| ENC000611 | 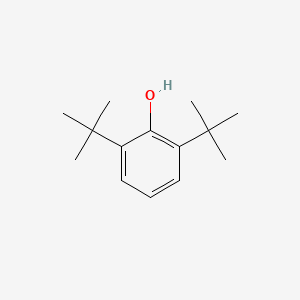 |
0.592 | D00NJL | 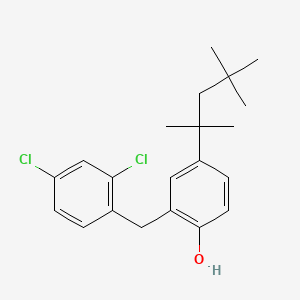 |
0.268 | ||
| ENC000658 | 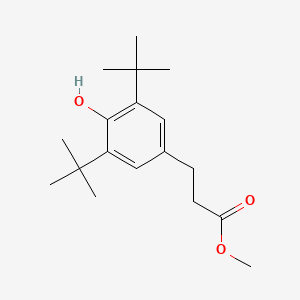 |
0.567 | D07EXH | 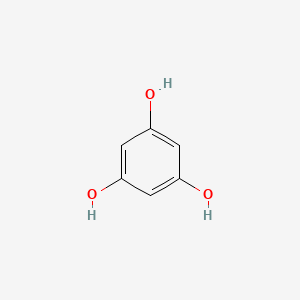 |
0.255 | ||
| ENC001398 | 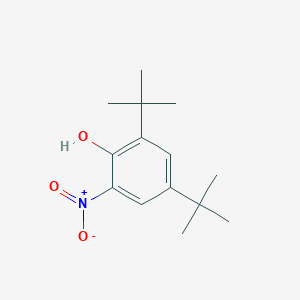 |
0.545 | D0Y4DY | 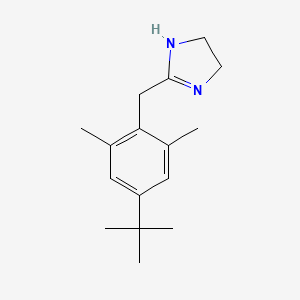 |
0.254 | ||
| ENC000695 | 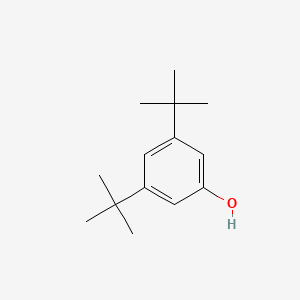 |
0.529 | D01JFT | 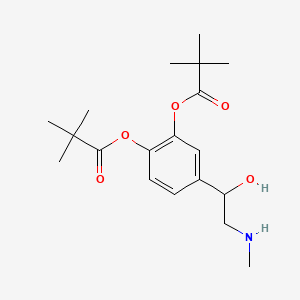 |
0.250 | ||
| ENC000185 | 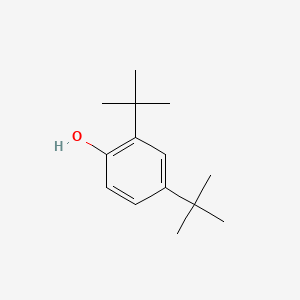 |
0.500 | D0X5NX | 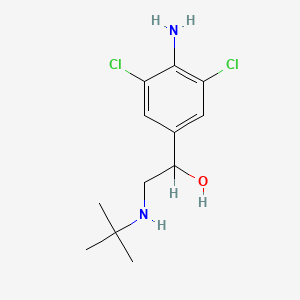 |
0.235 | ||
| ENC000744 | 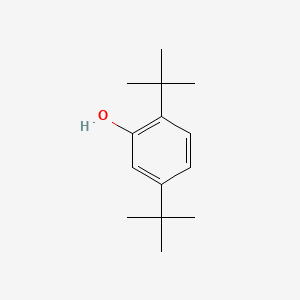 |
0.500 | D0K5CB | 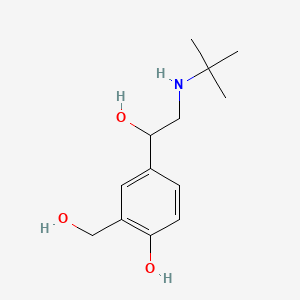 |
0.232 | ||