NPs Basic Information

|
Name |
4,5-Dimethylthiazole
|
| Molecular Formula | C5H7NS | |
| IUPAC Name* |
4,5-dimethyl-1,3-thiazole
|
|
| SMILES |
CC1=C(SC=N1)C
|
|
| InChI |
InChI=1S/C5H7NS/c1-4-5(2)7-3-6-4/h3H,1-2H3
|
|
| InChIKey |
UWSONZCNXUSTKW-UHFFFAOYSA-N
|
|
| Synonyms |
4,5-DIMETHYLTHIAZOLE; 3581-91-7; 4,5-Dimethyl-1,3-thiazole; Thiazole, 4,5-dimethyl-; 4,5-dimethyl thiazole; 4,5-dimethyl-thiazole; FEMA No. 3274; MFCD00005336; U3RP5I088G; EINECS 222-703-3; BRN 0105694; UNII-U3RP5I088G; dimethylthiazole, 4,5-; FMOC-D-LYS-OHHCL; 4-27-00-00986 (Beilstein Handbook Reference); 4,5-Dimethylthiazole, 97%; SCHEMBL104484; FEMA 3274; 4,5-Dimethyl-1,3-thiazole #; DTXSID40189348; CHEBI:193947; ZINC407027; AMY23262; 4,5-Dimethylthiazole, >=97%, FG; AKOS005207225; 4,5-DIMETHYL THIAZOLE [FHFI]; CS-W011256; (trichloromethyl)-Phosphonous dichloride; BP-10468; CS-17342; BB 0263152; D2142; FT-0617208; EN300-50400; A822988; Q-100428; Q27290649
|
|
| CAS | 3581-91-7 | |
| PubChem CID | 62510 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 113.18 | ALogp: | 1.8 |
| HBD: | 0 | HBA: | 2 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 41.1 | Aromatic Rings: | 1 |
| Heavy Atoms: | 7 | QED Weighted: | 0.503 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.34 | MDCK Permeability: | 0.00003940 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.003 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.462 |
| 30% Bioavailability (F30%): | 0.741 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.954 | Plasma Protein Binding (PPB): | 56.33% |
| Volume Distribution (VD): | 0.992 | Fu: | 49.11% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.853 | CYP1A2-substrate: | 0.917 |
| CYP2C19-inhibitor: | 0.106 | CYP2C19-substrate: | 0.834 |
| CYP2C9-inhibitor: | 0.012 | CYP2C9-substrate: | 0.409 |
| CYP2D6-inhibitor: | 0.209 | CYP2D6-substrate: | 0.498 |
| CYP3A4-inhibitor: | 0.017 | CYP3A4-substrate: | 0.483 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 9.804 | Half-life (T1/2): | 0.632 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.008 | Human Hepatotoxicity (H-HT): | 0.114 |
| Drug-inuced Liver Injury (DILI): | 0.669 | AMES Toxicity: | 0.266 |
| Rat Oral Acute Toxicity: | 0.067 | Maximum Recommended Daily Dose: | 0.029 |
| Skin Sensitization: | 0.294 | Carcinogencity: | 0.491 |
| Eye Corrosion: | 0.973 | Eye Irritation: | 0.994 |
| Respiratory Toxicity: | 0.769 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000646 | 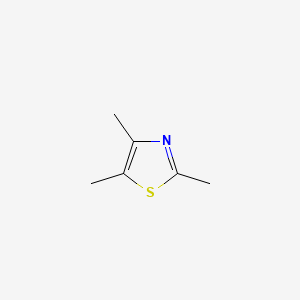 |
0.333 | D06PQT | 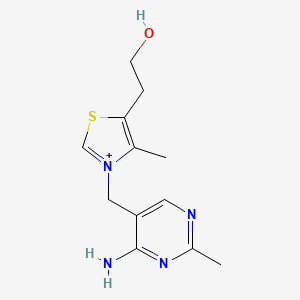 |
0.190 | ||
| ENC003532 | 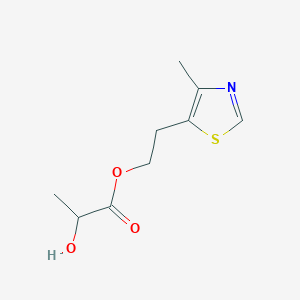 |
0.326 | D02LDV | 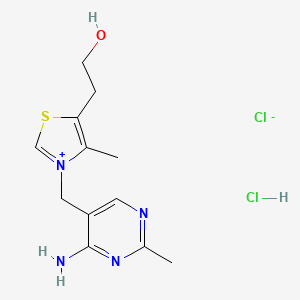 |
0.183 | ||
| ENC000599 |  |
0.281 | D07MUN |  |
0.182 | ||
| ENC000577 |  |
0.257 | D0V5IW |  |
0.178 | ||
| ENC000177 | 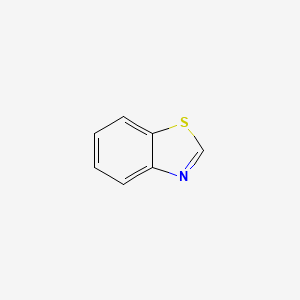 |
0.243 | D0S1NZ | 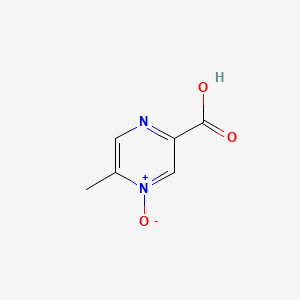 |
0.171 | ||
| ENC000240 |  |
0.242 | D0D7EN | 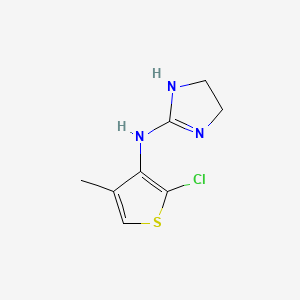 |
0.167 | ||
| ENC000092 | 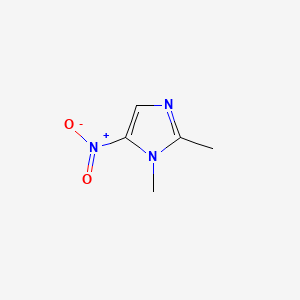 |
0.216 | D02NJA | 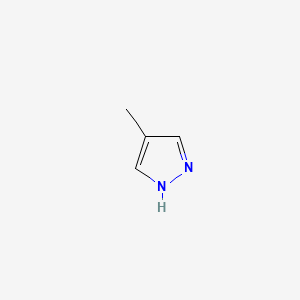 |
0.161 | ||
| ENC000477 | 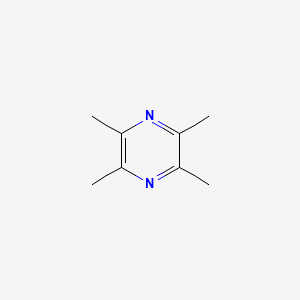 |
0.216 | D0A2ZX | 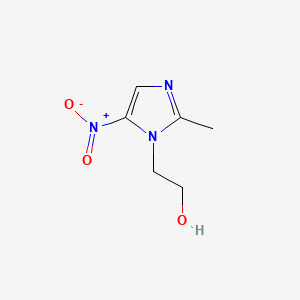 |
0.159 | ||
| ENC000179 | 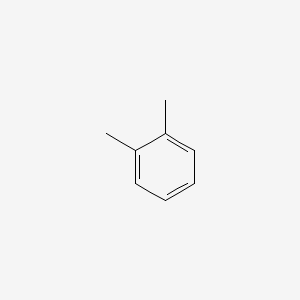 |
0.206 | D0N0OU | 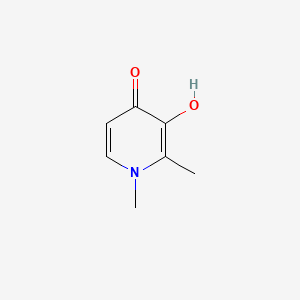 |
0.154 | ||
| ENC000239 | 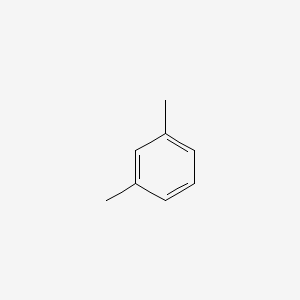 |
0.206 | D09TBD |  |
0.153 | ||