NPs Basic Information
|
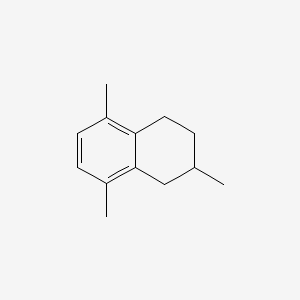
Name |
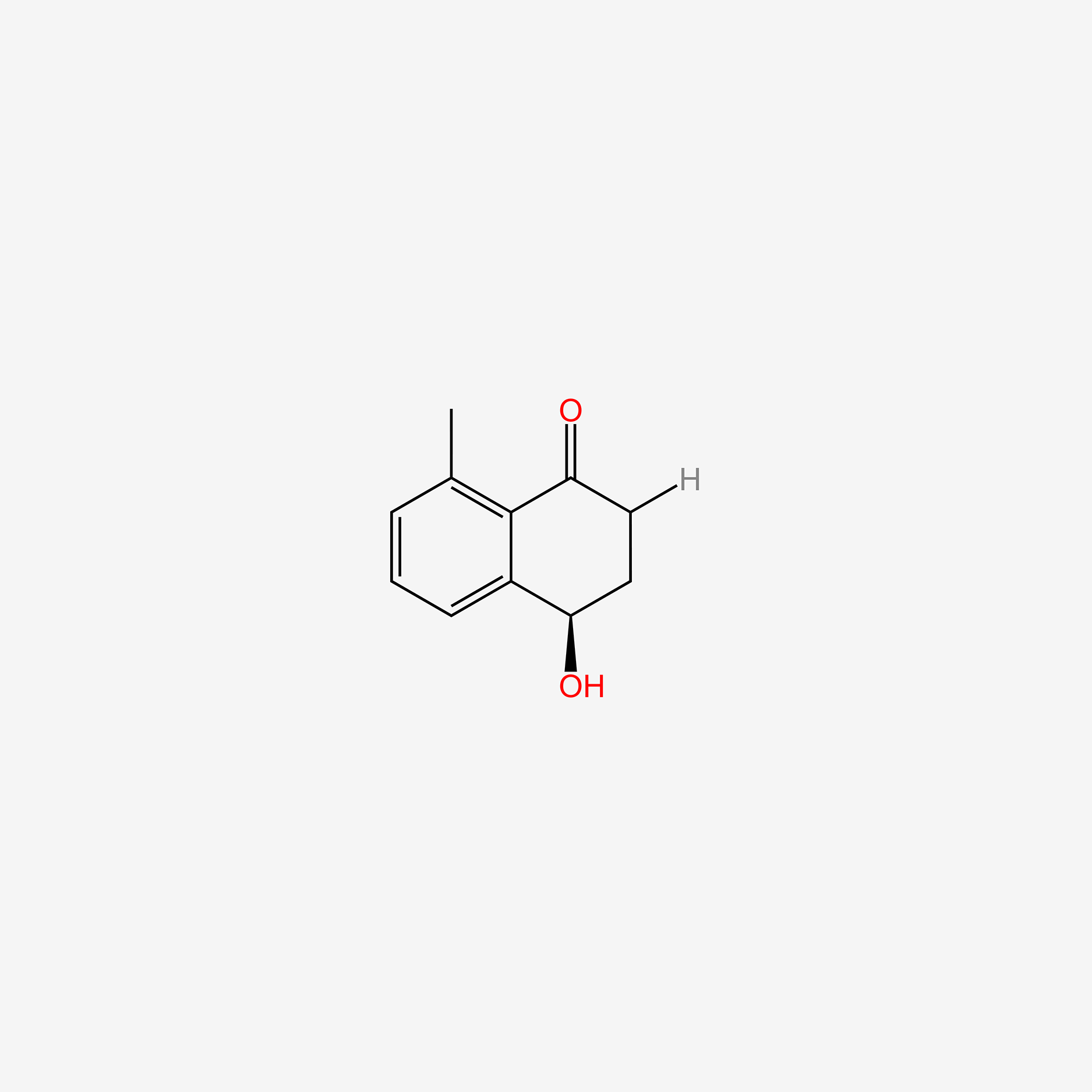
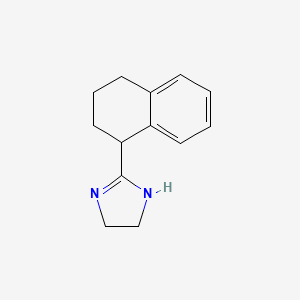
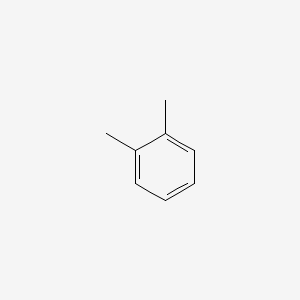
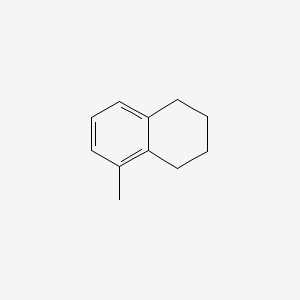
Naphthalene, 1,2,3,4-tetrahydro-5-methyl-
|
| Molecular Formula | C11H14 | |
| IUPAC Name* |
5-methyl-1,2,3,4-tetrahydronaphthalene
|
|
| SMILES |
CC1=C2CCCCC2=CC=C1
|
|
| InChI |
InChI=1S/C11H14/c1-9-5-4-7-10-6-2-3-8-11(9)10/h4-5,7H,2-3,6,8H2,1H3
|
|
| InChIKey |
YXOVIGZJPGLNGM-UHFFFAOYSA-N
|
|
| Synonyms |
2809-64-5; 5-METHYLTETRALINE; 5-Methyl-1,2,3,4-tetrahydronaphthalene; Naphthalene, 1,2,3,4-tetrahydro-5-methyl-; 5-METHYLTETRALIN; 888WK5BNU1; 1-Methyl-5,6,7,8-tetrahydronaphthalene; 1,2,3,4-Tetrahydro-5-methyl-naphthalene; UNII-888WK5BNU1; DTXSID50182365; MFCD00216193; ZINC95891302; AKOS006273345; 1,2,3,4-tetrahydro-5-methylnaphthalene; AS-56833; CS-0197111; FT-0705885; N10858; Q27269900
|
|
| CAS | 2809-64-5 | |
| PubChem CID | 17768 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Physi-Chem Properties
| Molecular Weight: | 146.23 | ALogp: | 3.7 |
| HBD: | 0 | HBA: | 0 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 0.0 | Aromatic Rings: | 2 |
| Heavy Atoms: | 11 | QED Weighted: | 0.524 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.467 | MDCK Permeability: | 0.00001880 |
| Pgp-inhibitor: | 0.045 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.002 | 20% Bioavailability (F20%): | 0.167 |
| 30% Bioavailability (F30%): | 0.963 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.922 | Plasma Protein Binding (PPB): | 94.39% |
| Volume Distribution (VD): | 1.951 | Fu: | 4.60% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.954 | CYP1A2-substrate: | 0.886 |
| CYP2C19-inhibitor: | 0.713 | CYP2C19-substrate: | 0.241 |
| CYP2C9-inhibitor: | 0.291 | CYP2C9-substrate: | 0.85 |
| CYP2D6-inhibitor: | 0.36 | CYP2D6-substrate: | 0.917 |
| CYP3A4-inhibitor: | 0.121 | CYP3A4-substrate: | 0.233 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.724 | Half-life (T1/2): | 0.343 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.034 | Human Hepatotoxicity (H-HT): | 0.056 |
| Drug-inuced Liver Injury (DILI): | 0.045 | AMES Toxicity: | 0.074 |
| Rat Oral Acute Toxicity: | 0.055 | Maximum Recommended Daily Dose: | 0.131 |
| Skin Sensitization: | 0.675 | Carcinogencity: | 0.298 |
| Eye Corrosion: | 0.879 | Eye Irritation: | 0.992 |
| Respiratory Toxicity: | 0.211 |