NPs Basic Information
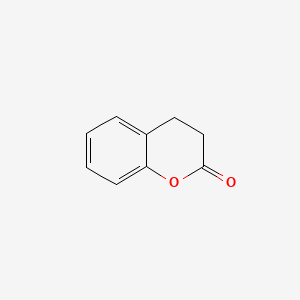
|
Name |
3,4-Dihydrocoumarin
|
| Molecular Formula | C9H8O2 | |
| IUPAC Name* |
3,4-dihydrochromen-2-one
|
|
| SMILES |
C1CC(=O)OC2=CC=CC=C21
|
|
| InChI |
InChI=1S/C9H8O2/c10-9-6-5-7-3-1-2-4-8(7)11-9/h1-4H,5-6H2
|
|
| InChIKey |
VMUXSMXIQBNMGZ-UHFFFAOYSA-N
|
|
| Synonyms |
dihydrocoumarin; 3,4-dihydrocoumarin; 119-84-6; hydrocoumarin; chroman-2-one; Benzodihydropyrone; melilotin; 2-chromanone; melilotol; 1,2-benzodihydropyrone; melilotic lactone; Melilotine; 2H-1-Benzopyran-2-one, 3,4-dihydro-; Oxochroman; 3,4-Dihydro-2H-1-benzopyran-2-one; Melilotic acid lactone; Chroman, 2-oxo-; Benzopyranone, dihydro-; Dihydrobenzopyrone; Coumarin, 3,4-dihydro-; Usaf do-12; 2-Hydroxydihydrocinnamic acid lactone; Meliotine; NCI-C55890; 3,4-dihydrochromen-2-one; 3,4-Dyhydrocoumarin; 3,4-Dihydro-1-benzopyran-2-one; 3,4-dihydro-2H-chromen-2-one; o-hydroxydihydrocinnamic acid lactone; FEMA No. 2381; 2-oxo-chroman; Hydrocinnamic acid, o-hydroxy-, delta-lactone; Hydroxydihydrocinnamic acid lactone, o-; o-hydroxyhydrocinnamic acid delta-lactone; o-hydroxyhydrocinnamic acid lactone; NM5K1Y1BT2; CHEMBL89306; COUMARIN,3,4-DIHYDRO; CHEBI:16151; NSC10121; NSC-10121; DSSTox_CID_474; Hydrocinnamic acid, o-hydroxy-, .delta.-lactone; DSSTox_RID_75613; DSSTox_GSID_20474; CAS-119-84-6; CCRIS 5803; HSDB 4333; EINECS 204-354-9; NSC 10121; UNII-NM5K1Y1BT2; BRN 0004584; dihydrocumarin; dihydrocoumarine; AI3-03425; 2-Oxochroman; Melilotin??; Dihydrobenzenopyrone; Hydrocoumarin, 8CI; Dihydro-Benzopyranone; MFCD00006881; 2,3-dihydrocoumarin; 3,4-Dihydrocumarine; Coumarin,4-dihydro-; Melilotin (coumarin); 3,4-dihydrocoumarine; hydrocinnamic acid, o-hydroxy-,lactone; 3,4 -dihydrocoumarin; 3,4-Dihydroxycoumarin; 3,4-Dihydro-Coumarin; Dihydrocoumarin, 99%; MELILOTIN [HSDB]; bmse000412; SCHEMBL28795; 5-17-10-00013 (Beilstein Handbook Reference); MLS002454372; 2-Hydroxyhydrocinnamic lactone; DIHYDROCOUMARIN [FHFI]; DIHYDROCOUMARIN [INCI]; DTXSID2020474; WLN: T66 BOVT & J; FEMA 2381; HMS2268K22; Dihydrocoumarin, analytical standard; HY-N1926; ZINC5934751; Hydrocinnamic acid, .delta.-lactone; Tox21_202137; Tox21_302745; 2H-1-Benzopyran-2-one,4-dihydro-; BBL027621; BDBM50146070; s9379; STK801851; Dihydrocoumarin, >=99%, FCC, FG; 1,2-BENZODIHYDROPYRONE [FCC]; AKOS000277415; CCG-266187; NCGC00091491-01; NCGC00091491-02; NCGC00256356-01; NCGC00259686-01; AC-34197; NCI60_000035; SMR000112325; VS-08570; 2H-1-Benzopyran-2-one, 3, 4-dihydro-; DB-061667; CS-0018237; D1223; FT-0614320; Dihydrocoumarin 100 microg/mL in Acetonitrile; EN300-49183; C02274; D70234; A892403; O-HYDROXY-HYDROCINNAMIC ACID-.DELTA.-LACTONE; W-108495; Q27098403; Z586249466
|
|
| CAS | 119-84-6 | |
| PubChem CID | 660 | |
| ChEMBL ID | CHEMBL89306 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 148.16 | ALogp: | 1.6 |
| HBD: | 0 | HBA: | 2 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 26.3 | Aromatic Rings: | 2 |
| Heavy Atoms: | 11 | QED Weighted: | 0.416 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.501 | MDCK Permeability: | 0.00003030 |
| Pgp-inhibitor: | 0.023 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.004 |
| 30% Bioavailability (F30%): | 0.146 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.868 | Plasma Protein Binding (PPB): | 77.27% |
| Volume Distribution (VD): | 0.465 | Fu: | 21.99% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.964 | CYP1A2-substrate: | 0.147 |
| CYP2C19-inhibitor: | 0.713 | CYP2C19-substrate: | 0.167 |
| CYP2C9-inhibitor: | 0.152 | CYP2C9-substrate: | 0.743 |
| CYP2D6-inhibitor: | 0.008 | CYP2D6-substrate: | 0.762 |
| CYP3A4-inhibitor: | 0.017 | CYP3A4-substrate: | 0.24 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 10.207 | Half-life (T1/2): | 0.83 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.008 | Human Hepatotoxicity (H-HT): | 0.021 |
| Drug-inuced Liver Injury (DILI): | 0.047 | AMES Toxicity: | 0.069 |
| Rat Oral Acute Toxicity: | 0.045 | Maximum Recommended Daily Dose: | 0.079 |
| Skin Sensitization: | 0.943 | Carcinogencity: | 0.796 |
| Eye Corrosion: | 0.931 | Eye Irritation: | 0.979 |
| Respiratory Toxicity: | 0.373 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000681 | 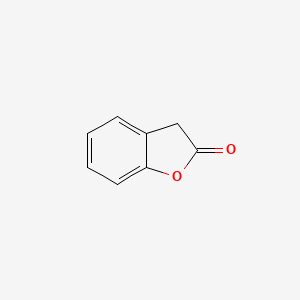 |
0.694 | D0MP5H | 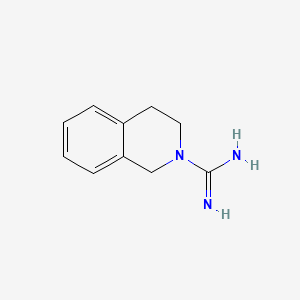 |
0.327 | ||
| ENC000673 | 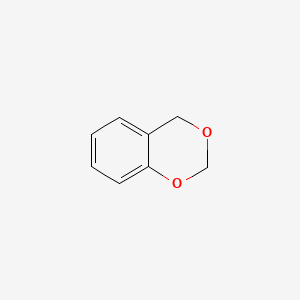 |
0.442 | D06OMW | 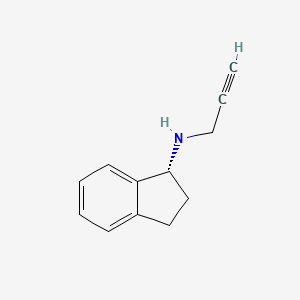 |
0.321 | ||
| ENC000345 | 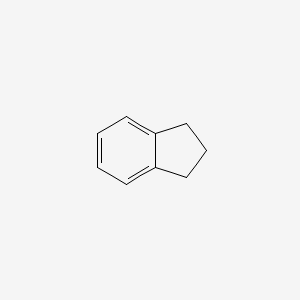 |
0.439 | D0UM7O | 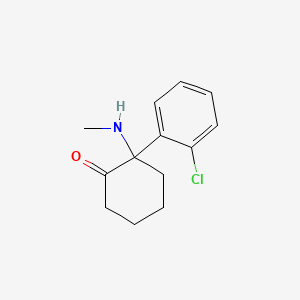 |
0.305 | ||
| ENC004792 | 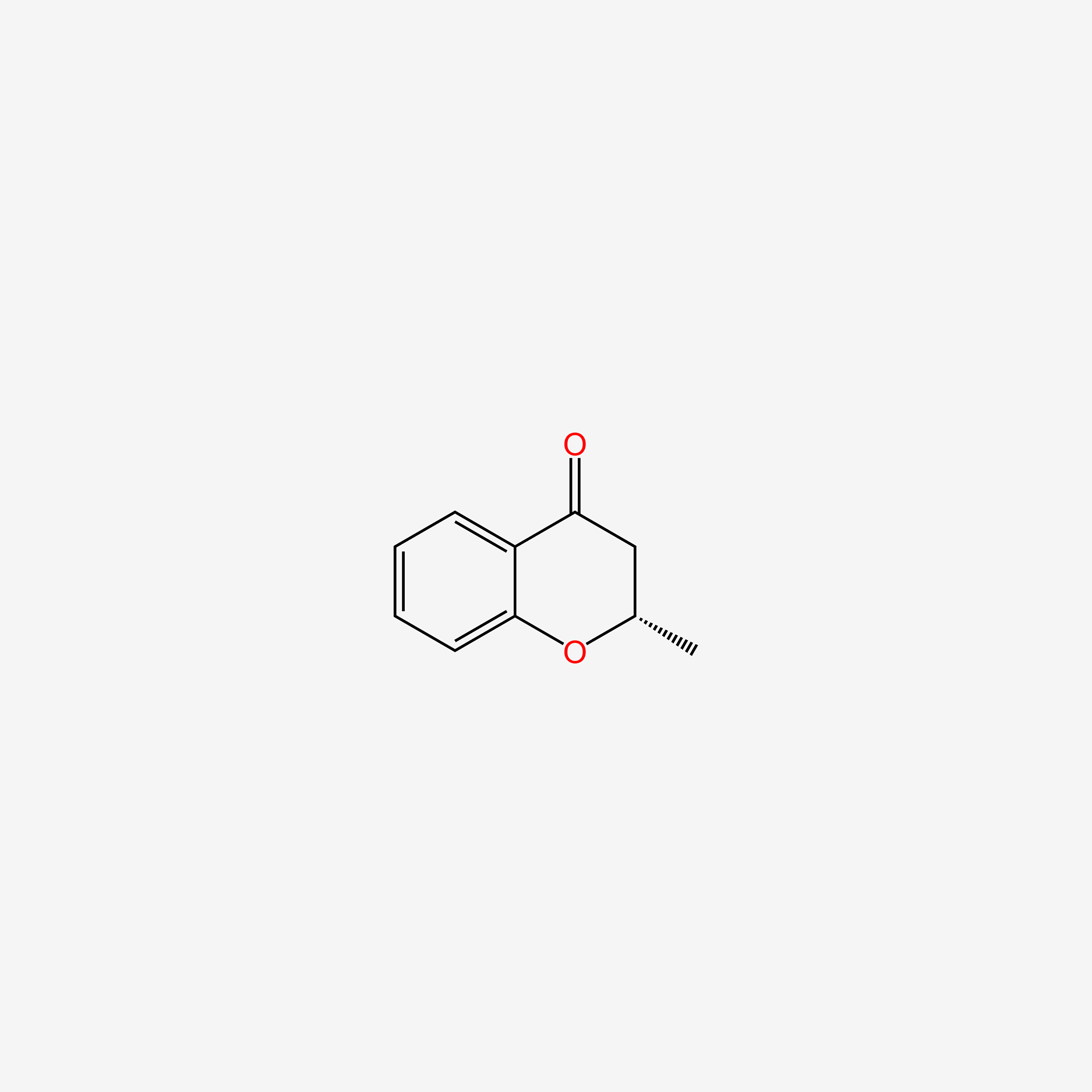 |
0.404 | D05IHU | 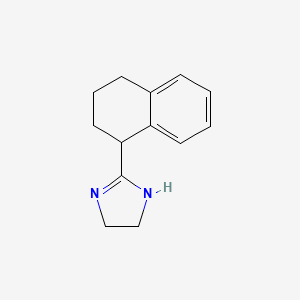 |
0.305 | ||
| ENC002236 |  |
0.400 | D0Z9NZ | 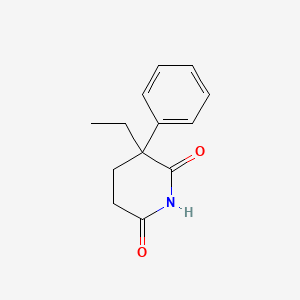 |
0.305 | ||
| ENC000171 | 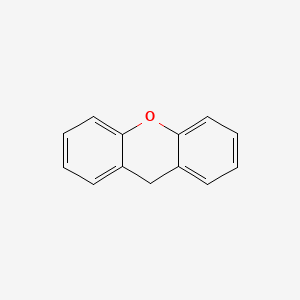 |
0.396 | D0R8PX | 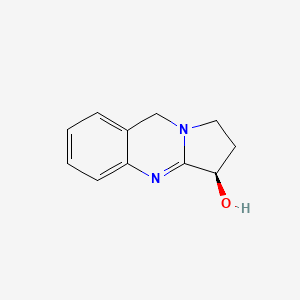 |
0.304 | ||
| ENC001380 | 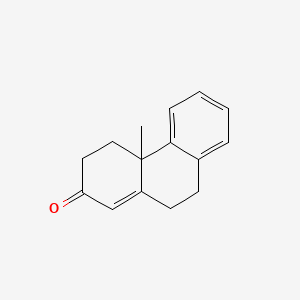 |
0.393 | D06DLI | 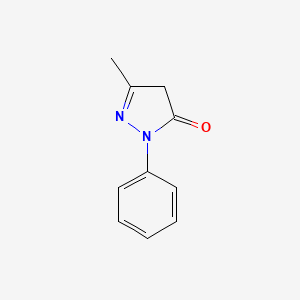 |
0.302 | ||
| ENC005244 | 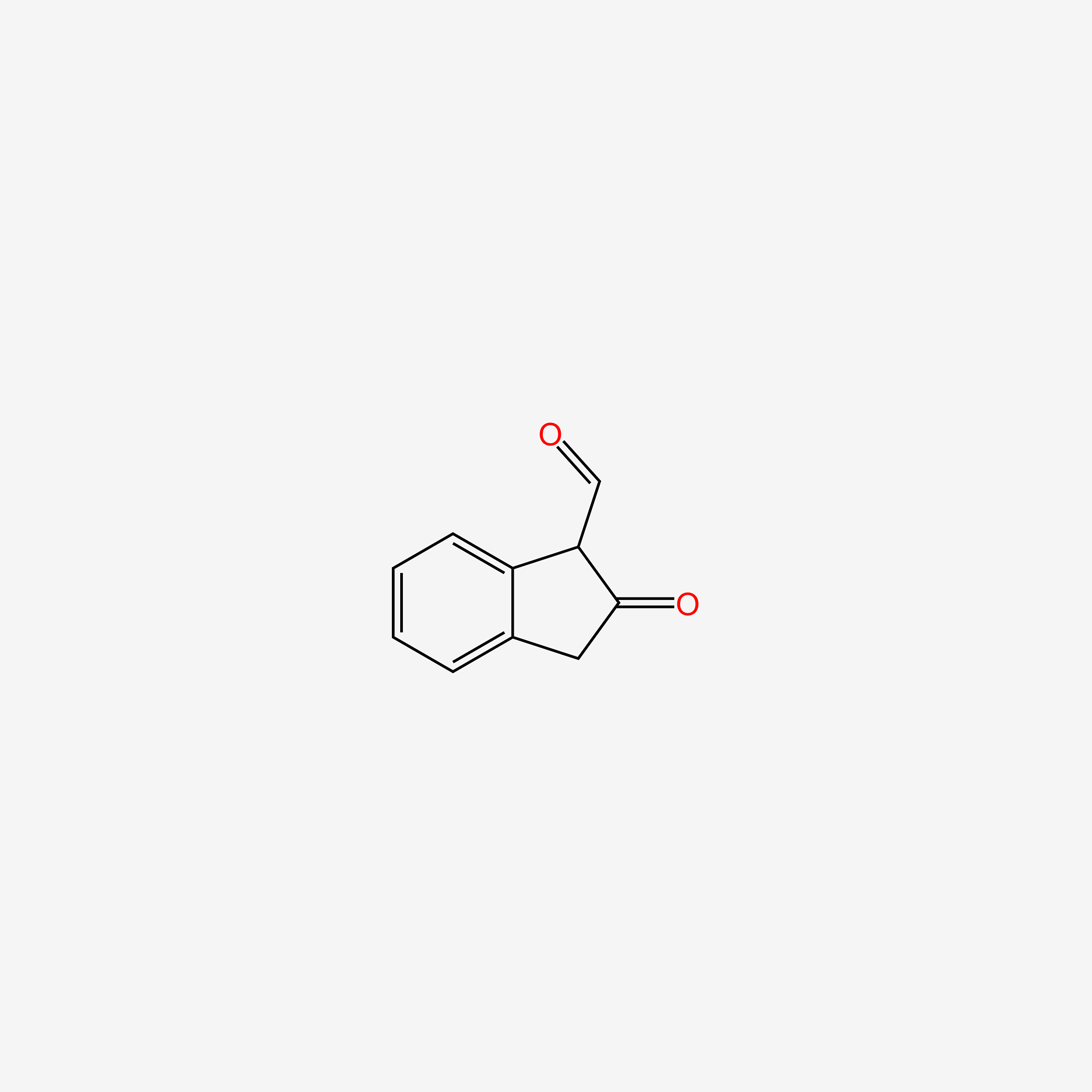 |
0.375 | D0D5GG | 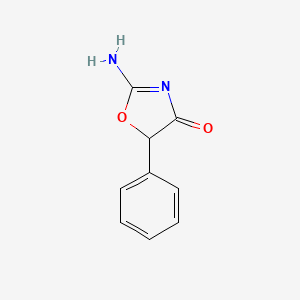 |
0.302 | ||
| ENC006142 | 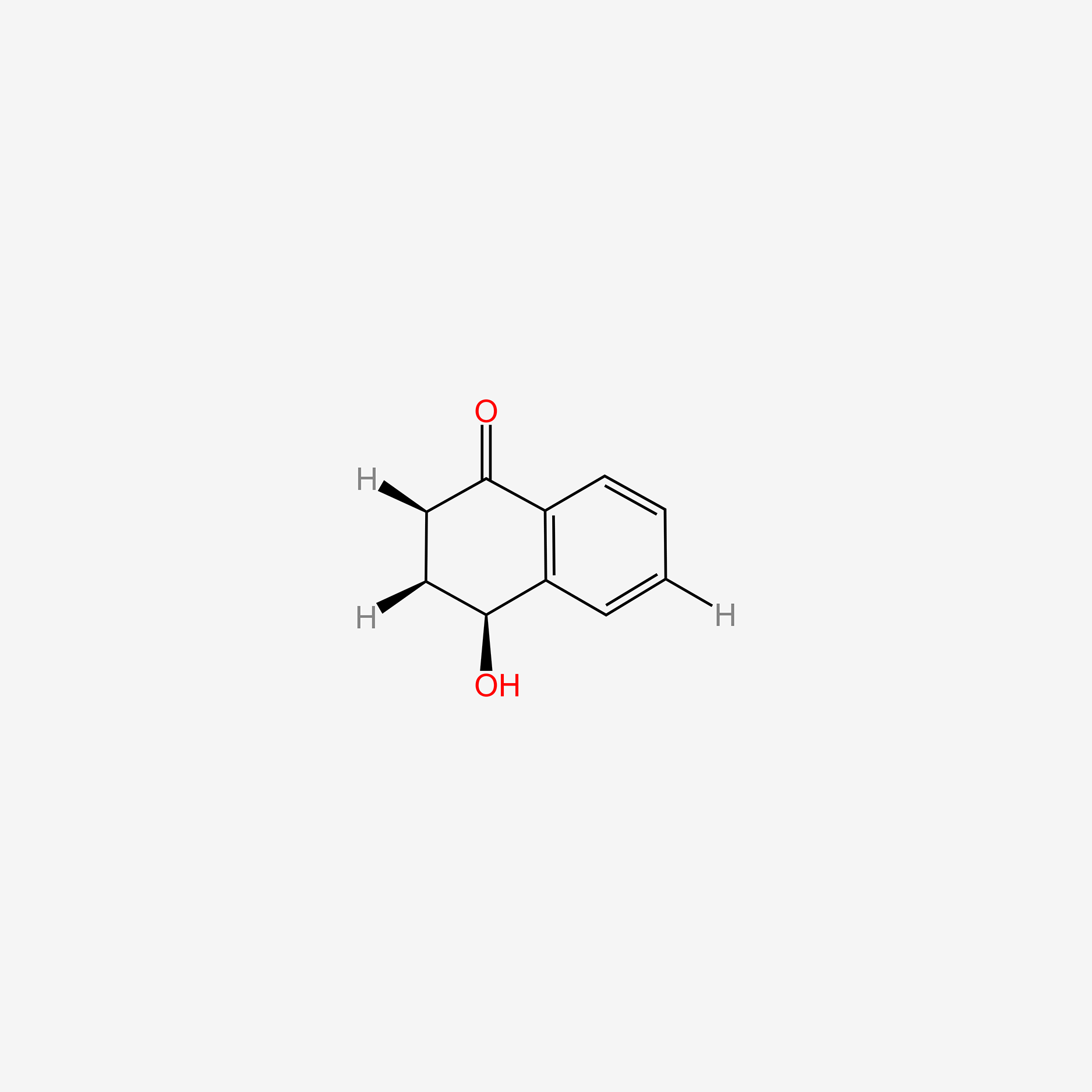 |
0.375 | D06BYV | 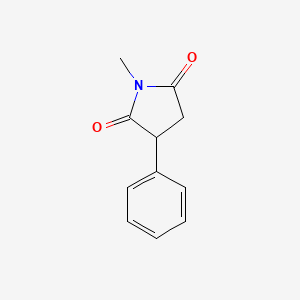 |
0.291 | ||
| ENC002076 | 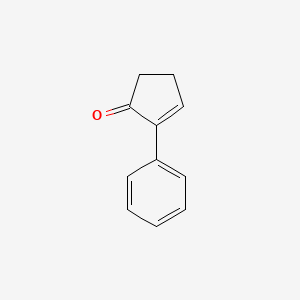 |
0.367 | D0E6YQ | 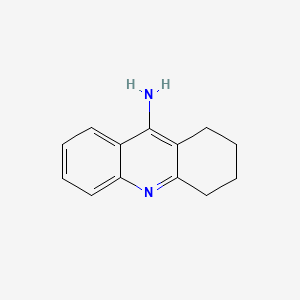 |
0.288 | ||