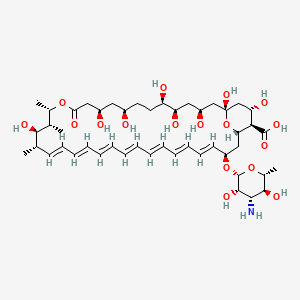
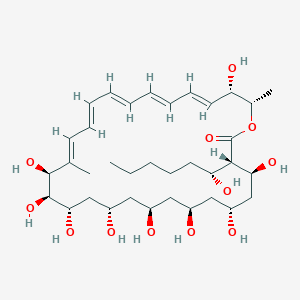
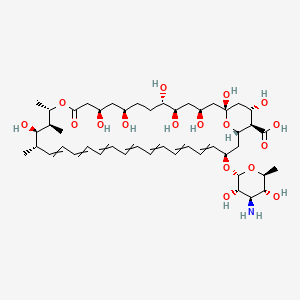
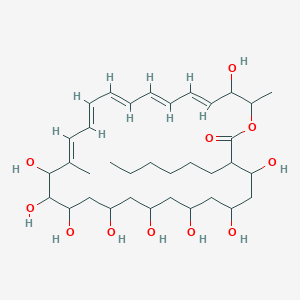
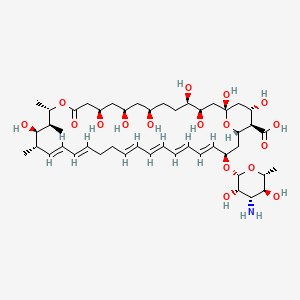
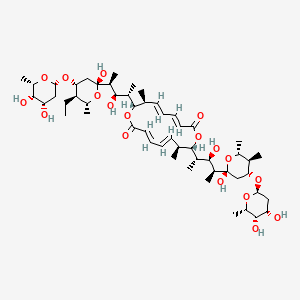
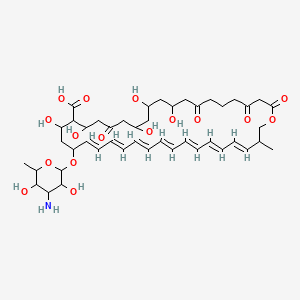
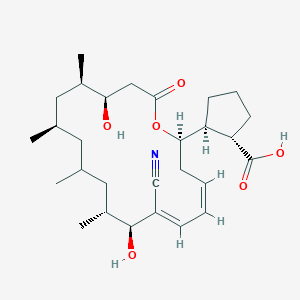
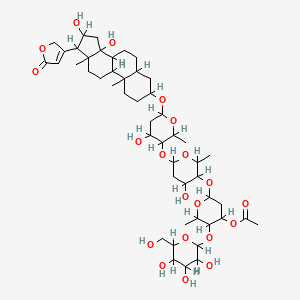
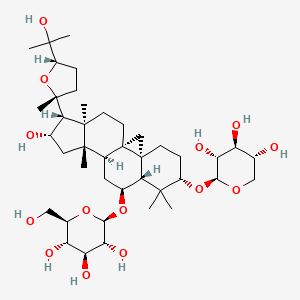
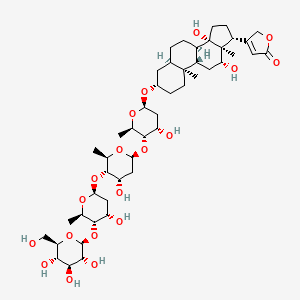
| Synonyms |
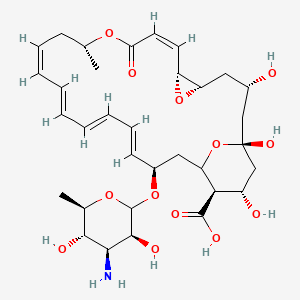
amphotericin b; Amphotericin; 1397-89-3; Ambisome; Amphotericine B; Fungizone; Halizon; Ampho-Moronal; Amphotericinum B; Liposomal Amphotericin B; Amfotericina B; AMPH-B; Abelcet; Amphotericin-B; Amphotec; Fungilin; Amphotocerin; Ambil; Amphotericin B liposome; amphotericin B liposomal; MFCD00877763; 7XU7A7DROE; NSC 527017; CHEBI:2682; ABELCET, LIPOSOMAL AMPHOTERICIN B; NCGC00090808-01; DSSTox_CID_2601; NSC-527017; DSSTox_RID_76653; DSSTox_GSID_22601; ABLC; Abelecet; Amphortericin B; Anfotericine B; (1R,3S,5R,6R,9R,11R,15S,16R,17R,18S,19E,21E,23E,25E,27E,29E,31E,33R,35S,36R,37S)-33-(((2R,3S,4S,5S,6R)-4-amino-3,5-dihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-1,3,5,6,9,11,17,37-octahydroxy-15,16,18-trimethyl-13-oxo-14,39-dioxabicyclo[33.3.1]nonatriaconta-19,21,23,25,27,29,31-heptaene-36-carboxylic acid; (1R,3S,5R,6R,9R,11R,15S,16R,17R,18S,19E,21E,23E,25E,27E,29E,31E,33R,35S,36R,37S)-33-[(3-amino-3,6-dideoxy-beta-D-mannopyranosyl)oxy]-1,3,5,6,9,11,17,37-octahydroxy-15,16,18-trimethyl-13-oxo-14,39-dioxabicyclo[33.3.1]nonatriaconta-19,21,23,25,27,29,31-heptaene-36-carboxylic acid; Amphotericin B trihydrate; IAB; UNII-7XU7A7DROE; Fungisome; Amfotericina B [INN-Spanish]; Amphotericine B [INN-French]; Amphotericinum B [INN-Latin]; CCRIS 5963; HSDB 3008; Amphotericin B [USP:INN:JAN]; Fungizone (TN); Amphotec (TN); (1S,3R,4E,6E,8E,10E,12E,14E,16E,18S,19R,20R,21S,25R,27R,30R,31R,33S,35R,37S,38R)-3-[(2R,3S,4S,5S,6R)-4-amino-3,5-dihydroxy-6-methyl-tetrahydropyran-2-yl]oxy-19,25,27,30,31,33,35,37-octahydroxy-18,20,21-trimethyl-23-oxo-22,39-dioxabicyclo[33.3.1]nonatriaconta-4,6,8,10,12,14,16-heptaene-38-carboxylic acid; AmBisome (TN); Amp B; CAS-1397-89-3; EINECS 215-742-2; NS 718; BRN 0078342; Amphotericin b, liposome; AI3-26528; Prestwick3_000410; Amphotericin B (85%); Amphotericin B solubilized; AMPHOTERICIN B [MI]; SCHEMBL17973; AMPHOTERICIN B [INN]; AMPHOTERICIN B [JAN]; BSPBio_000340; 5-18-10-00525 (Beilstein Handbook Reference); AMPHOTERICIN B [HSDB]; BIDD:GT0351; AMPHOTERICIN B [VANDF]; BPBio1_000374; CHEMBL267345; NKTR-024; AMPHOTERICIN B [MART.]; AMPHOTERICIN B [USP-RS]; AMPHOTERICIN B [WHO-DD]; AMPHOTERICIN B [WHO-IP]; DTXSID9022601; HMS2096A22; HMS3713A22; Amphotericin B (JP17/USP/INN); HY-B0221; Amphotericin b, liposome [WHO-DD]; Amphotericin B, Streptomyces nodosus; Tox21_111027; Tox21_202484; AMPHOTERICIN B [ORANGE BOOK]; LMPK06000002; s1636; AMPHOTERICIN B [EP MONOGRAPH]; AKOS024464746; AMPHOTERICIN B [USP MONOGRAPH]; ZINC253387843; AMPHOTERICIN B LIPOSOME [VANDF]; CCG-220410; DB00681; AMPHOTERICINUM B [WHO-IP LATIN]; NCGC00260033-01; (1R-(1R*,3S*,5R*,6R*,9R*,11R*,15S*,16R*,17R*,18S*,19E,21E,23E,25E,27E,29E,31E,33R*,35S*,36R*,37S*))-33-((3-Amino-3,6-dideoxy-beta-D-mannopyranosyl)oxy)-1,3,5,6,9,11,17,37-octahydroxy-15,16,18-trimethyl-13-oxo-14,39-dioxabicyclo(33.3.1)nonatriaconta-19,21,23,25,27,29,31-heptaene-36-carboxylic acid; 14,39-Dioxabicyclo(33.3.1)nonatriaconta-19,21,23,25,2 7,29,31-heptaene-36-carboxylic acid, 33-((3-amino-3,6-dideoxy-beta-D-mannopyranosyl)oxy)-1,3,5,6,9,11,17,37-octahydroxy-15,16,18-trimethyl-13-oxo-(1R-(1R*,3S*,5R*,6R*,9R*,11R*,15S*,16R*,17R*,18S*,19E,21E,23E, 25E-27E,29E,31E,33R*,35S*,36R*,37S*))-; AB00513832; C06573; D00203; AB00513832_02; 397A893; Q412223; 1397-89-3, C47H73NO17; Amphotericin B from Streptomyces sp., ~80% (HPLC), powder; Amphotericin B from Streptomyces sp., BioReagent, suitable for cell culture, ~80% (HPLC); Amphotericin B solubilized, powder, gamma-irradiated, BioXtra, suitable for cell culture; (1R,3S,5R,6R,9R,11R,15S,16R,17R,18S,19E,21E,23E,25E,27E,29E,31E,33R,35S,36R,37S)-33-[(2R,3S,4S,5S,6R)-4-amino-3,5-dihydroxy-6-methyloxan-2-yl]oxy-1,3,5,6,9,11,17,37-octahydroxy-15,16,18-trimethyl-13-oxo-14,39-dioxabicyclo[33.3.1]nonatriaconta-19,21,23,25,; (1R,3S,5R,6R,9R,11R,15S,16R,17R,18S,19E,21E,23E,25E,27E,29E,31E,33R,35S,36R,37S)-33-[(2R,3S,4S,5S,6R)-4-amino-3,5-dihydroxy-6-methyloxan-2-yl]oxy-1,3,5,6,9,11,17,37-octahydroxy-15,16,18-trimethyl-13-oxo-14,39-dioxabicyclo[33.3.1]nonatriaconta-19,21,23,25,27,29,31-heptaene-36-carboxylic acid; (1R,3S,5R,6R,9R,11R,15S,16R,17R,18S,19E,21E,23E,25E,27E,29E,31E,33R,35S,36R,37S)-33-[(3-Amino-3,6-dideoxy-?-D-mannopyranosyl)oxy]-1,3,5,6,9,11,17,37-octahydroxy-15,16,18-trimethyl-13-oxo-14,39-dioxabicyclo[33.3.1]nonatriaconta-19,21,23,25,27,29,31-heptaene-36-carboxylic acid; (1R,3S,5R,6R,9R,11R,15S,16R,17R,18S,19E,21E,23E,25E,27E,29E,31E,33R,35S,36R,37S)-33-[(3-amino-3,6-dideoxy-beta-D-mannopyranosyl)oxy]-1,3,5,6,9,11,17,37-octahydroxy-15,16,18-trimethyl-13-oxo-14,39-dioxabicyclo[33.3.1]nonatriaconta-19,21,23,25,27,29,31-he; (1R,3S,5R,6R,9R,11R,15S,16R,17R,18S,19E,21E,23E,25E,27E,29E,31E,33R,35S,36R,37S)-33-[(3-amino-3,6-dideoxy-beta-D-mannopyranosyl)oxy]-1,3,5,6,9,11,17,37-octahydroxy-15,16,18-trimethyl-13-oxo-14,39-dioxabicyclo[33.3.1]nonatriaconta-19,21,23,25,27,29,31-hept; (1R,3S,5R,6R,9R,11R,15S,16R,17R,18S,33R,35S,36R,37S)-33-{[(2R,3S,4S,5S,6R)-4-amino-3,5-dihydroxy-6-methyloxan-2-yl]oxy}-1,3,5,6,9,11,17,37-octahydroxy-15,16,18-trimethyl-13-oxo-14,39-dioxabicyclo[33.3.1]nonatriaconta-19,21,23,25,27,29,31-heptaene-36-carbo; (1S,3R,4E,6E,8E,10E,12E,14E,16E,18S,19R,20R,21S,25R,27R,30R,31R,33S,35R,37S,38R)-3-[(2R,3S,4S,5S,6R)-4-amino-3,5-dihydroxy-6-methyloxan-2-yl]oxy-19,25,27,30,31,33,35,37-octahydroxy-18,20,21-trimethyl-; 23-oxo-22,39-dioxabicyclo[33.3.1]nonatriaconta-4,6,8,10,12,14,16-heptaene-38-carboxylic acid; Amphotericin B from Streptomyces sp., Vetec(TM) reagent grade, BioReagent, suitable for cell culture, ~80%
|