NPs Basic Information
|
Name |
tert-Butylhydroquinone
|
| Molecular Formula | C10H14O2 | |
| IUPAC Name* |
2-tert-butylbenzene-1,4-diol
|
|
| SMILES |
CC(C)(C)C1=C(C=CC(=C1)O)O
|
|
| InChI |
InChI=1S/C10H14O2/c1-10(2,3)8-6-7(11)4-5-9(8)12/h4-6,11-12H,1-3H3
|
|
| InChIKey |
BGNXCDMCOKJUMV-UHFFFAOYSA-N
|
|
| Synonyms |
tert-Butylhydroquinone; 1948-33-0; TBHQ; 2-tert-Butylhydroquinone; 2-tert-butylbenzene-1,4-diol; T-BUTYLHYDROQUINONE; MTBHQ; t-Butyl hydroquinone; 2-t-Butylhydroquinone; Mono-tert-butylhydroquinone; 2-tert-Butyl-1,4-benzenediol; Sustane; Mono-tertiarybutylhydroquinone; tert-Butyl-1,4-benzenediol; Tenox TBHQ; tertiary-Butylhydroquinone; 1,4-Benzenediol, 2-(1,1-dimethylethyl)-; Hydroquinone, tert-butyl-; Banox 20BA; 2-(1,1-Dimethylethyl)-1,4-benzenediol; 2-tert-Butyl(1,4)hydroquinone; 2-tertiary-butylhydroquinone; t-BHQ; 2-(tert-butyl)benzene-1,4-diol; Tertiary butylhydroquinone; tert-Butylhydrochinone; 2-t-Butyl-1,4-benzenediol; Butylhydroquinone, tert-; Eastman MTBHQ; NSC 4972; MFCD00002344; 1,4-Benzenediol (1,1-dimethylethyl)-; CHEBI:78886; tert-butyl-hydroquinone; NSC4972; NSC-4972; 2-(1,1-dimethylethyl)benzene-1,4-diol; NCGC00013051-05; E319; DSSTox_CID_220; C12674942B; DSSTox_RID_75441; DSSTox_GSID_20220; Hydroquinone, t-butyl-; EYK; CAS-1948-33-0; monotertiary butyl hydroquinone; CCRIS 1447; HSDB 838; Butylhydroquinone, t-; Tenox 20; tert-butyl hydroquinone; EINECS 217-752-2; BRN 0637923; 2-(tert-Butyl)benzene-1,4-diol(may occur to produce black solid); AI3-61039; Sustane TBHQ; Tenox TBHO; UNII-C12674942B; Tenox TBHQTBHQ; t-butyl-hydroquinone; tert-Butylhydroquinon; 2-t-butyl hydroquinone; tert.-butyl hydroquinone; 2-tert-butyl-hydroquinone; TBHQ [INCI]; TBHQ [FCC]; EC 217-752-2; NCIStruc1_000241; NCIStruc2_000017; SCHEMBL26745; tert-Butylhydroquinone, 97%; MLS002222348; BUTYLHYDROQUINONE,TERT-; Mono-Tertiarybuytl Hydroquinone; CHEMBL242080; INS NO.319; DTXSID6020220; INS-319; NCI4972; HYDROQUINONE,TERTIARY BUTYL; WLN: QR DQ BX1&1&1; KUC109743N; T-BUTYLHYDROQUINONE [HSDB]; ZINC388085; 1,4-dihydroxy-2-tert-butylbenzene; 2-tert-butyl-1,4-dihydroxybenzene; TERT-BUTYLHYDROQUINONE [II]; Tox21_110006; Tox21_110007; Tox21_202309; Tox21_300081; BDBM50065387; CCG-37948; NCGC00013051; s4990; STK372011; AKOS003627061; CS-5774; DB07726; NCGC00013051-01; NCGC00013051-02; NCGC00013051-03; NCGC00013051-04; NCGC00013051-06; NCGC00013051-07; NCGC00013051-08; NCGC00013051-09; NCGC00013051-10; NCGC00090788-01; NCGC00090788-02; NCGC00090788-03; NCGC00090788-04; NCGC00254178-01; NCGC00259858-01; AC-10579; BS-15862; NCI60_004196; SMR001253806; SY001798; TERTIARY BUTYLHYDROQUINONE [MART.]; HYDROQUINONE,TERTIARY BUTYL [VANDF]; KSC-241-078-1; tert-Butylhydroquinone, analytical standard; DB-019879; HY-100489; B0833; E-319; FT-0652102; 2-(1,1-Dimethylethyl)-1,4-benzenediol, 9CI; D70420; EN300-120878; Q662443; W-107698; BRD-K36452089-001-01-8; BRD-K36452089-001-02-6; F0001-0696; Z1255402624
|
|
| CAS | 1948-33-0 | |
| PubChem CID | 16043 | |
| ChEMBL ID | CHEMBL242080 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 166.22 | ALogp: | 2.8 |
| HBD: | 2 | HBA: | 2 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 40.5 | Aromatic Rings: | 1 |
| Heavy Atoms: | 12 | QED Weighted: | 0.581 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.644 | MDCK Permeability: | 0.00002080 |
| Pgp-inhibitor: | 0.026 | Pgp-substrate: | 0.079 |
| Human Intestinal Absorption (HIA): | 0.006 | 20% Bioavailability (F20%): | 0.957 |
| 30% Bioavailability (F30%): | 0.934 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.077 | Plasma Protein Binding (PPB): | 90.72% |
| Volume Distribution (VD): | 2.609 | Fu: | 13.58% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.879 | CYP1A2-substrate: | 0.899 |
| CYP2C19-inhibitor: | 0.495 | CYP2C19-substrate: | 0.274 |
| CYP2C9-inhibitor: | 0.4 | CYP2C9-substrate: | 0.937 |
| CYP2D6-inhibitor: | 0.789 | CYP2D6-substrate: | 0.887 |
| CYP3A4-inhibitor: | 0.144 | CYP3A4-substrate: | 0.368 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 12.666 | Half-life (T1/2): | 0.899 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.011 | Human Hepatotoxicity (H-HT): | 0.056 |
| Drug-inuced Liver Injury (DILI): | 0.03 | AMES Toxicity: | 0.021 |
| Rat Oral Acute Toxicity: | 0.619 | Maximum Recommended Daily Dose: | 0.542 |
| Skin Sensitization: | 0.925 | Carcinogencity: | 0.113 |
| Eye Corrosion: | 0.982 | Eye Irritation: | 0.978 |
| Respiratory Toxicity: | 0.454 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
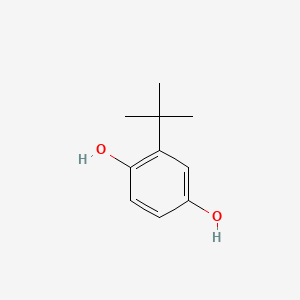
| ENC000185 | 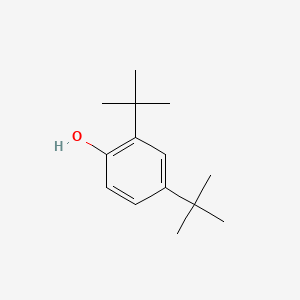 |
0.568 | D0K5CB | 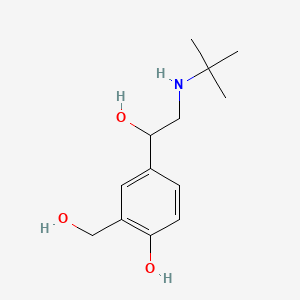 |
0.382 | ||
| ENC005113 | 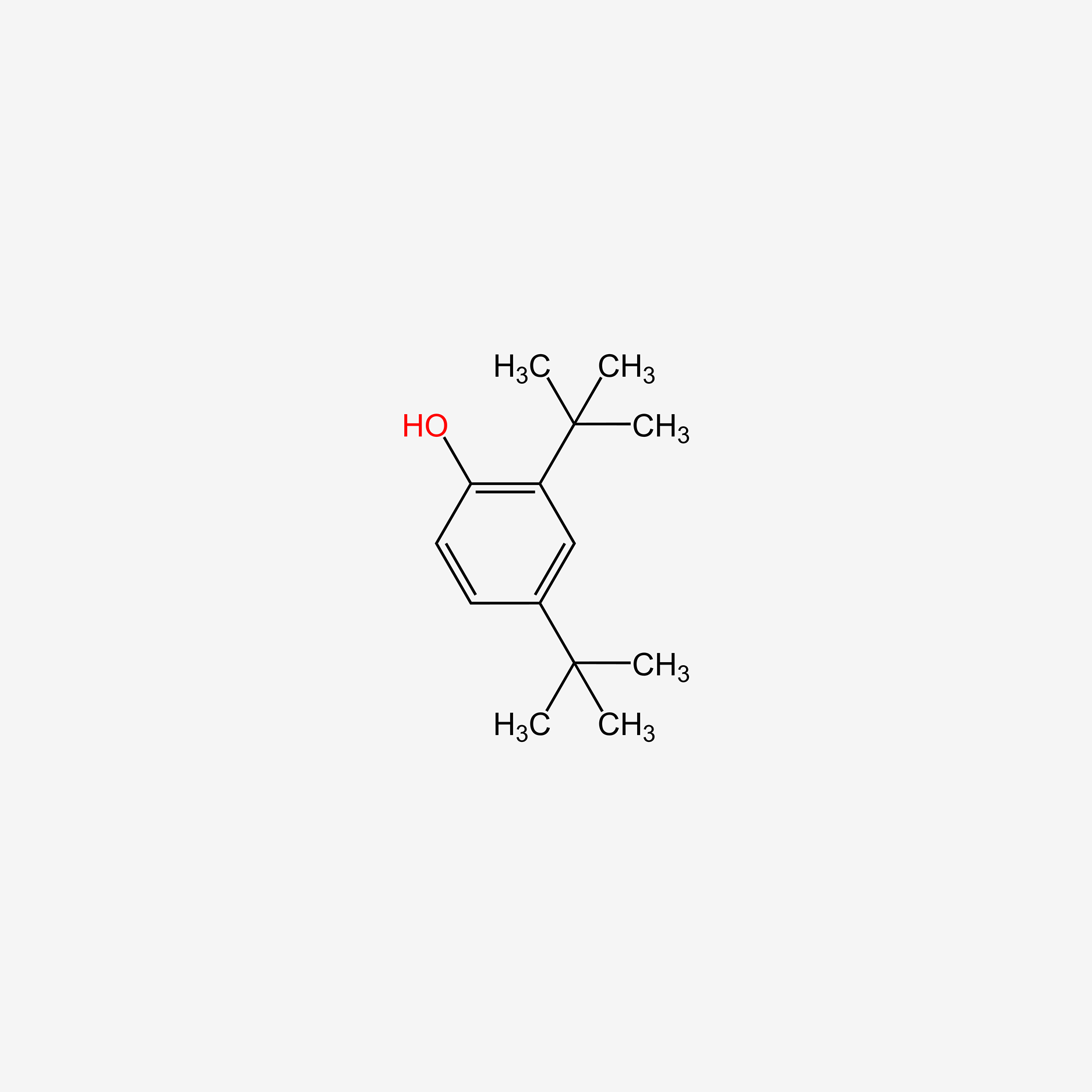 |
0.568 | D02ZJI | 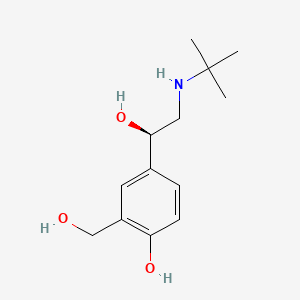 |
0.382 | ||
| ENC000394 | 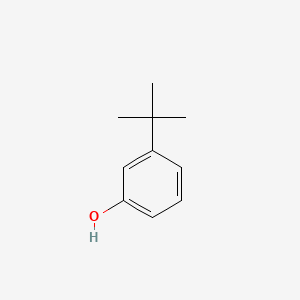 |
0.500 | D0YF3X | 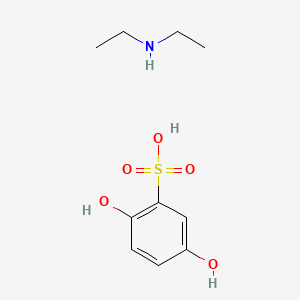 |
0.364 | ||
| ENC000744 | 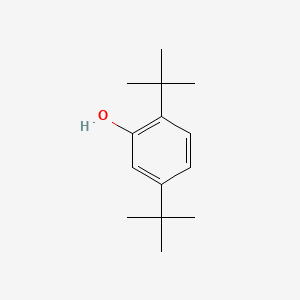 |
0.468 | D0M8RC | 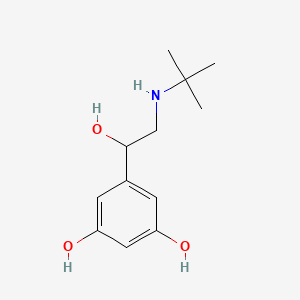 |
0.352 | ||
| ENC000344 | 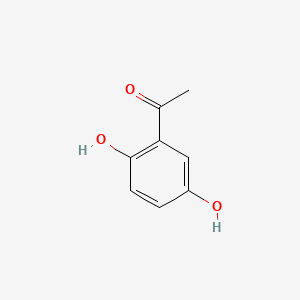 |
0.463 | D0BA6T | 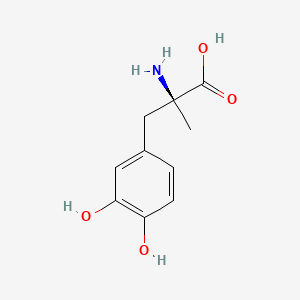 |
0.346 | ||
| ENC000696 | 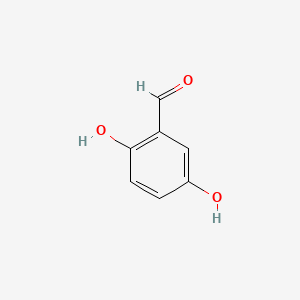 |
0.450 | D06GIP |  |
0.333 | ||
| ENC000985 |  |
0.450 | D0SS4P |  |
0.333 | ||
| ENC000725 |  |
0.449 | D0Y6KO | 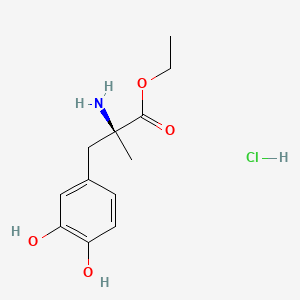 |
0.328 | ||
| ENC000079 | 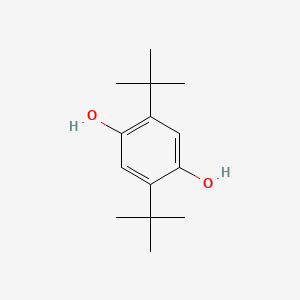 |
0.449 | D0P7JZ | 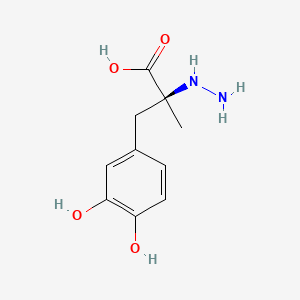 |
0.327 | ||
| ENC000097 | 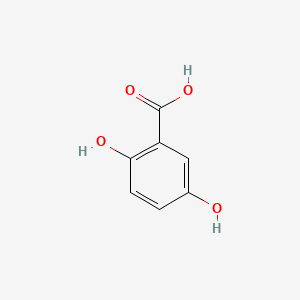 |
0.429 | D0T7OW | 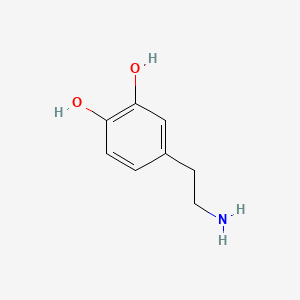 |
0.326 | ||