NPs Basic Information
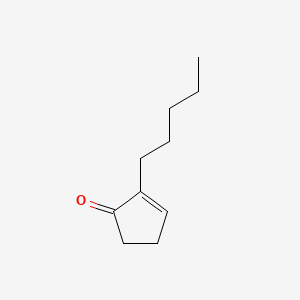
|
Name |
2-Pentyl-2-cyclopenten-1-one
|
| Molecular Formula | C10H16O | |
| IUPAC Name* |
2-pentylcyclopent-2-en-1-one
|
|
| SMILES |
CCCCCC1=CCCC1=O
|
|
| InChI |
InChI=1S/C10H16O/c1-2-3-4-6-9-7-5-8-10(9)11/h7H,2-6,8H2,1H3
|
|
| InChIKey |
ILHZVKAXFCDFMT-UHFFFAOYSA-N
|
|
| Synonyms |
2-Pentyl-2-cyclopenten-1-one; 25564-22-1; 2-Pentylcyclopent-2-en-1-one; 2-Cyclopenten-1-one, 2-pentyl-; Amyl cyclopentenone; 2-Pentyl-2-Cyclopentene-1-One; 2-pentyl-2-cyclopentenone; 1UOK9N9N72; 2-pentyl-cyclopent-2-en-1-one; DSSTox_CID_9331; DSSTox_RID_78766; DSSTox_GSID_29331; 2-pentylcyclopent-2-enone; CAS-25564-22-1; BRN 1857975; EINECS 247-104-4; UNII-1UOK9N9N72; EC 247-104-4; 2-pentyl-cyclopent-2-enone; 2-n-pentyl-2-cyclopentenone; 4-07-00-00195 (Beilstein Handbook Reference); 2-Amyl 2-cyclopenten-1-one; SCHEMBL357488; CHEMBL3183509; DTXSID6029331; 2-Cyclopenten-1-one,2-pentyl-; 2-n-pentyl-cyclopent-2-en-1-one; ZINC2077861; Tox21_201738; Tox21_303448; BBL009816; MFCD00036631; STL145849; AKOS000120813; NCGC00249109-01; NCGC00257294-01; NCGC00259287-01; VS-02198; DB-049940; CS-0152312; FT-0711591; P1142; EN300-18643; D84134; Q27252921
|
|
| CAS | 25564-22-1 | |
| PubChem CID | 117549 | |
| ChEMBL ID | CHEMBL3183509 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 152.23 | ALogp: | 3.0 |
| HBD: | 0 | HBA: | 1 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 17.1 | Aromatic Rings: | 1 |
| Heavy Atoms: | 11 | QED Weighted: | 0.562 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.489 | MDCK Permeability: | 0.00001950 |
| Pgp-inhibitor: | 0.245 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.036 |
| 30% Bioavailability (F30%): | 0.065 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.991 | Plasma Protein Binding (PPB): | 83.92% |
| Volume Distribution (VD): | 0.575 | Fu: | 25.48% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.845 | CYP1A2-substrate: | 0.89 |
| CYP2C19-inhibitor: | 0.554 | CYP2C19-substrate: | 0.41 |
| CYP2C9-inhibitor: | 0.384 | CYP2C9-substrate: | 0.707 |
| CYP2D6-inhibitor: | 0.081 | CYP2D6-substrate: | 0.421 |
| CYP3A4-inhibitor: | 0.055 | CYP3A4-substrate: | 0.203 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 5.326 | Half-life (T1/2): | 0.592 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.027 | Human Hepatotoxicity (H-HT): | 0.582 |
| Drug-inuced Liver Injury (DILI): | 0.042 | AMES Toxicity: | 0.038 |
| Rat Oral Acute Toxicity: | 0.209 | Maximum Recommended Daily Dose: | 0.823 |
| Skin Sensitization: | 0.954 | Carcinogencity: | 0.759 |
| Eye Corrosion: | 0.932 | Eye Irritation: | 0.986 |
| Respiratory Toxicity: | 0.654 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000617 | 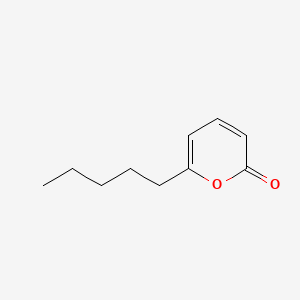 |
0.383 | D01QLH | 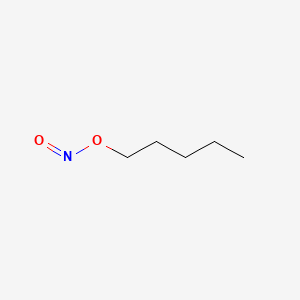 |
0.293 | ||
| ENC004625 | 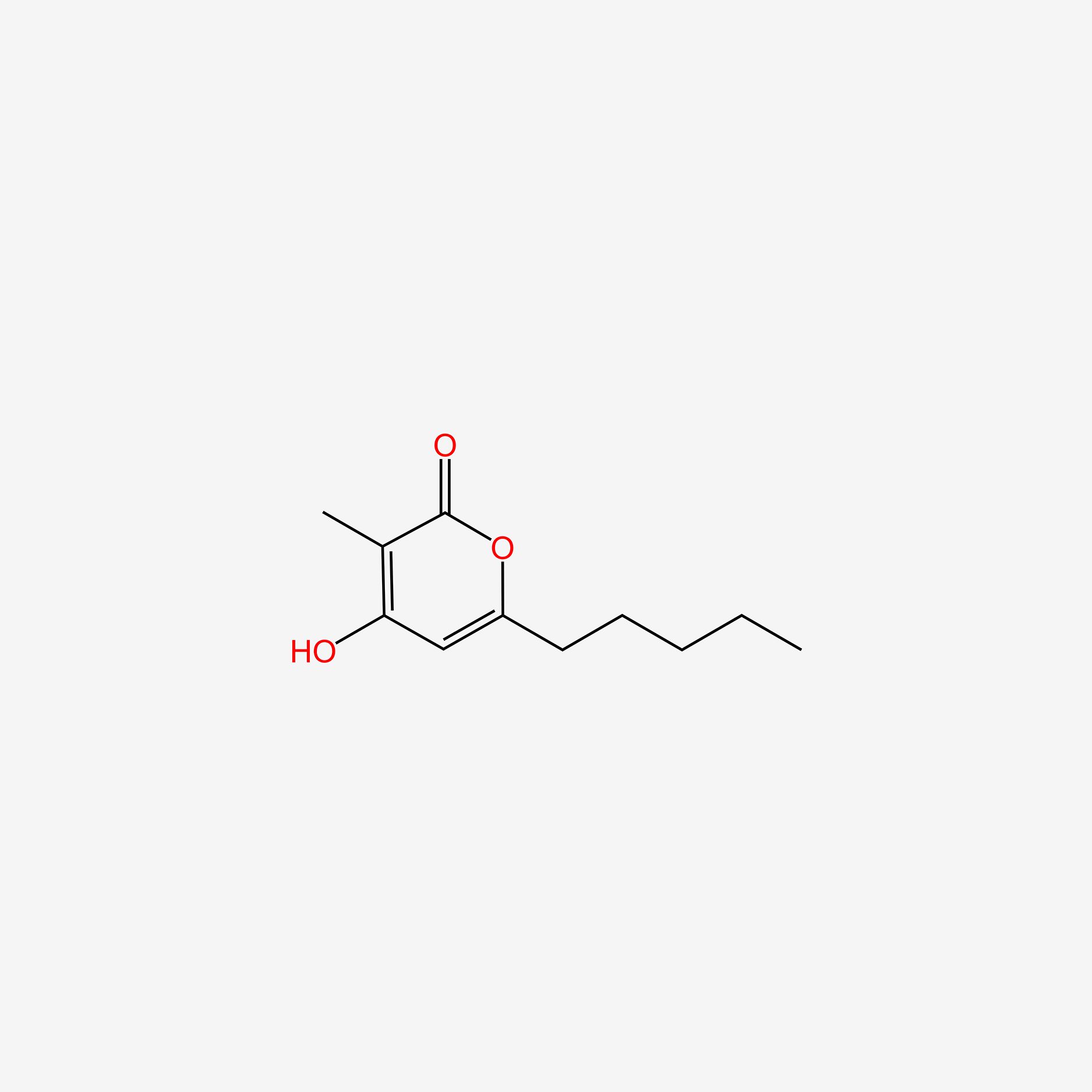 |
0.353 | D0O1UZ | 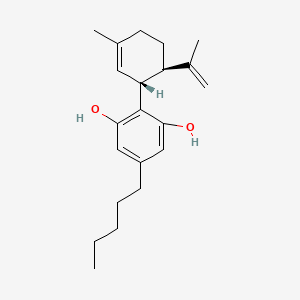 |
0.253 | ||
| ENC000899 | 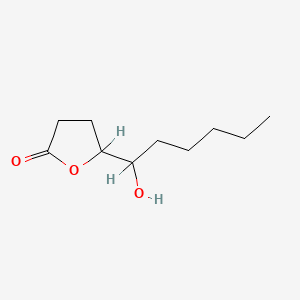 |
0.340 | D0P1FO | 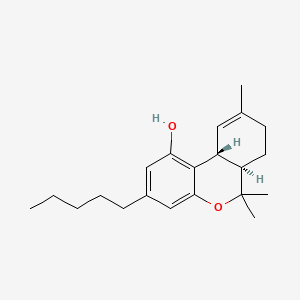 |
0.250 | ||
| ENC000533 | 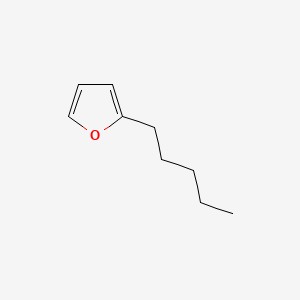 |
0.333 | D0O3AB | 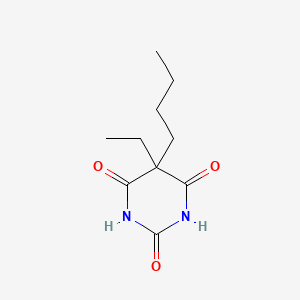 |
0.224 | ||
| ENC001195 | 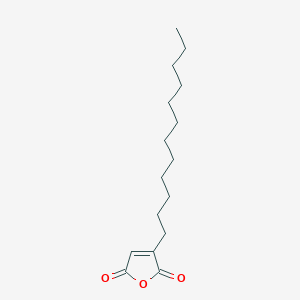 |
0.308 | D0L7AS | 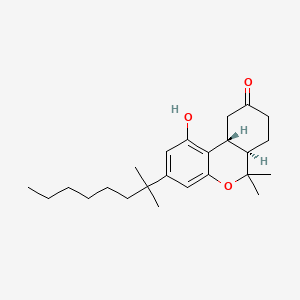 |
0.221 | ||
| ENC000139 | 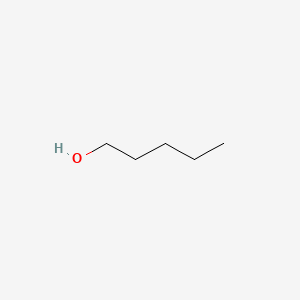 |
0.306 | D00MIN | 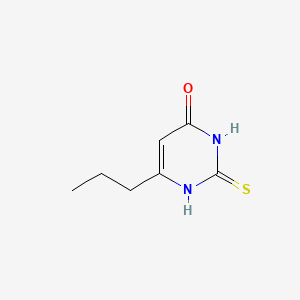 |
0.220 | ||
| ENC000315 | 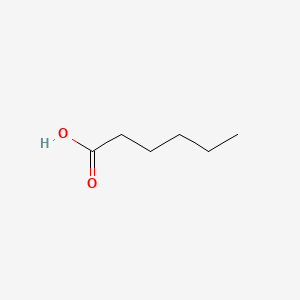 |
0.300 | D03ZJE | 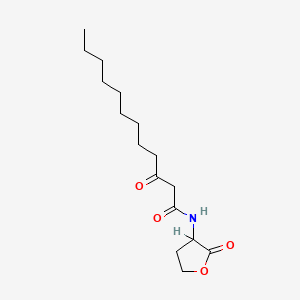 |
0.216 | ||
| ENC000250 | 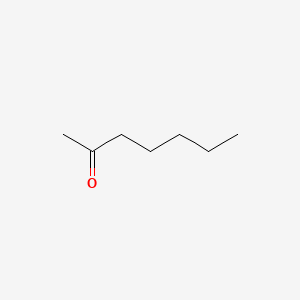 |
0.300 | D07UHS |  |
0.208 | ||
| ENC005831 | 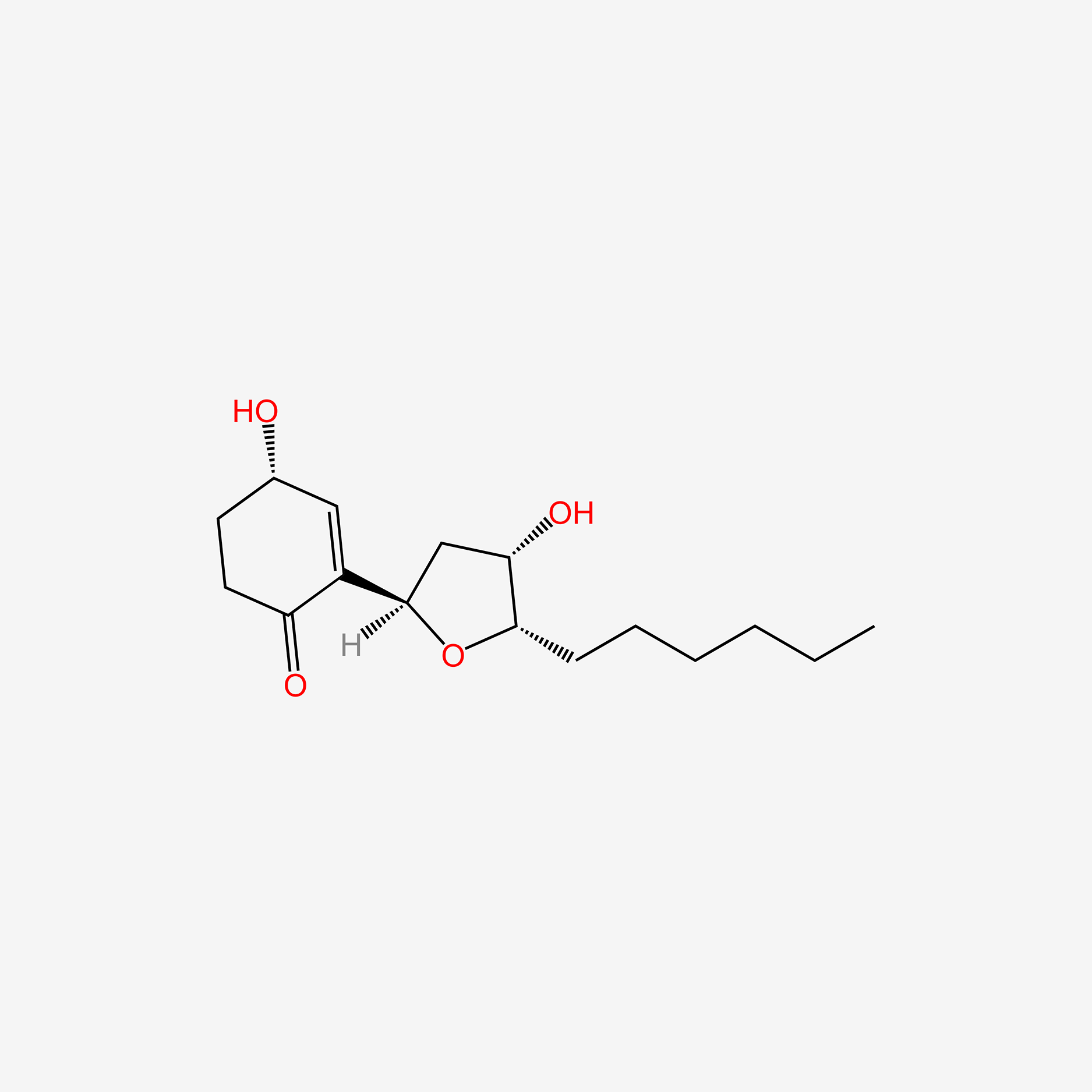 |
0.299 | D02HXS | 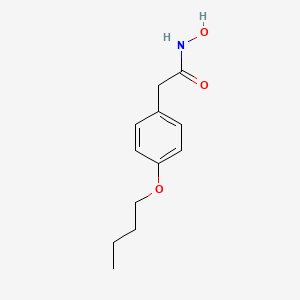 |
0.206 | ||
| ENC004248 | 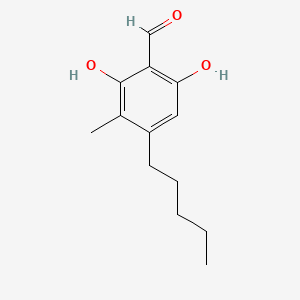 |
0.298 | D0R9EQ | 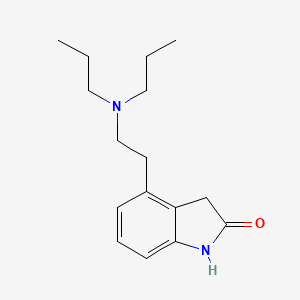 |
0.197 | ||