NPs Basic Information
|
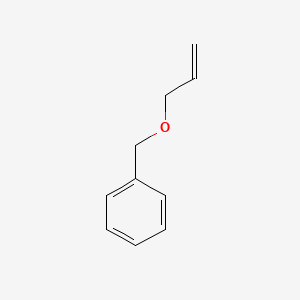
Name |
Allyl benzyl ether
|
| Molecular Formula | C10H12O | |
| IUPAC Name* |
prop-2-enoxymethylbenzene
|
|
| SMILES |
C=CCOCC1=CC=CC=C1
|
|
| InChI |
InChI=1S/C10H12O/c1-2-8-11-9-10-6-4-3-5-7-10/h2-7H,1,8-9H2
|
|
| InChIKey |
HUGHWHMUUQNACD-UHFFFAOYSA-N
|
|
| Synonyms |
Allyl benzyl ether; 14593-43-2; ((Allyloxy)methyl)benzene; prop-2-enoxymethylbenzene; Benzene, [(2-propenyloxy)methyl]-; alpha-(Allyloxy)toluene; Allylbenzyl ether; Benzyl allyl ether; Benzene, ((2-propenyloxy)methyl)-; BQZ35CE79E; [(prop-2-en-1-yloxy)methyl]benzene; MFCD00078288; Ether, allyl benzyl; EINECS 238-638-9; (allyloxymethyl)benzene; allyloxy-methyl-benzene; AI3-07338; Allyl benzyl ether, 99%; UNII-BQZ35CE79E; [(Allyloxy)methyl]benzene #; C6H5CH2OCH2CH=CH2; SCHEMBL149658; CHEMBL3905781; DTXSID3074529; SCHEMBL13158965; AMY5442; 3-(BENZYLOXY)-1-PROPENE; BDBM188218; ZINC2555302; CS1050; AKOS015889627; Benzene, [(2-propen-1-yloxy)methyl]-; AS-62579; SY052476; ((2-PROPEN-1-YLOXY)METHYL)BENZENE; DB-097384; CS-0157968; FT-0694811; EN300-92204; US9073941, 34; J-008172; Q63408739
|
|
| CAS | 14593-43-2 | |
| PubChem CID | 84542 | |
| ChEMBL ID | CHEMBL3905781 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 148.2 | ALogp: | 2.6 |
| HBD: | 0 | HBA: | 1 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 9.2 | Aromatic Rings: | 1 |
| Heavy Atoms: | 11 | QED Weighted: | 0.47 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.198 | MDCK Permeability: | 0.00005340 |
| Pgp-inhibitor: | 0.016 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.002 | 20% Bioavailability (F20%): | 0.515 |
| 30% Bioavailability (F30%): | 0.967 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.831 | Plasma Protein Binding (PPB): | 77.96% |
| Volume Distribution (VD): | 1.233 | Fu: | 15.62% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.563 | CYP1A2-substrate: | 0.305 |
| CYP2C19-inhibitor: | 0.526 | CYP2C19-substrate: | 0.243 |
| CYP2C9-inhibitor: | 0.107 | CYP2C9-substrate: | 0.099 |
| CYP2D6-inhibitor: | 0.03 | CYP2D6-substrate: | 0.31 |
| CYP3A4-inhibitor: | 0.055 | CYP3A4-substrate: | 0.305 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.774 | Half-life (T1/2): | 0.827 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.021 | Human Hepatotoxicity (H-HT): | 0.035 |
| Drug-inuced Liver Injury (DILI): | 0.024 | AMES Toxicity: | 0.083 |
| Rat Oral Acute Toxicity: | 0.197 | Maximum Recommended Daily Dose: | 0.035 |
| Skin Sensitization: | 0.911 | Carcinogencity: | 0.124 |
| Eye Corrosion: | 0.762 | Eye Irritation: | 0.993 |
| Respiratory Toxicity: | 0.064 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000308 | 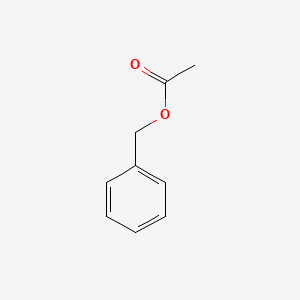 |
0.500 | D05OIS |  |
0.486 | ||
| ENC000596 | 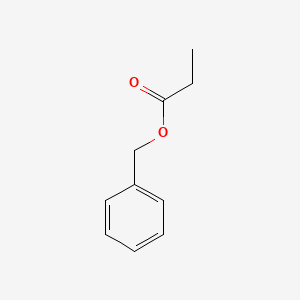 |
0.500 | D0P9AC | 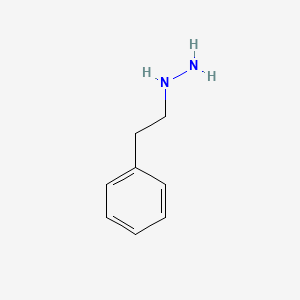 |
0.452 | ||
| ENC000128 | 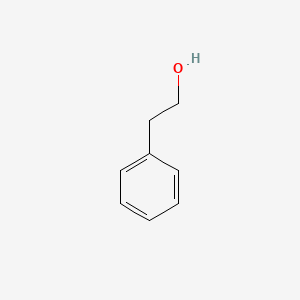 |
0.487 | D05BMG |  |
0.429 | ||
| ENC000217 | 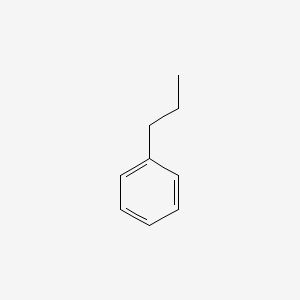 |
0.487 | D0T3LF | 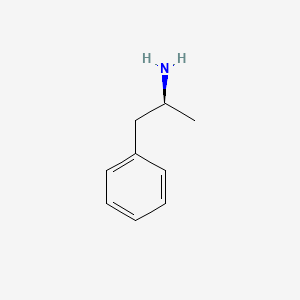 |
0.429 | ||
| ENC000053 | 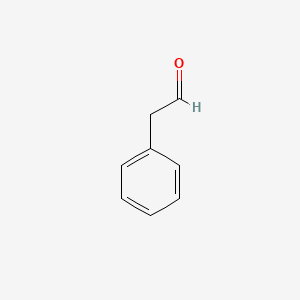 |
0.487 | D0U0RZ | 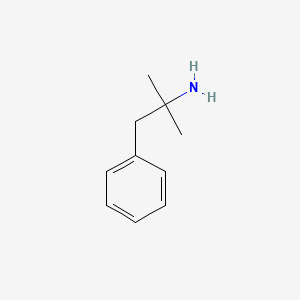 |
0.409 | ||
| ENC000205 | 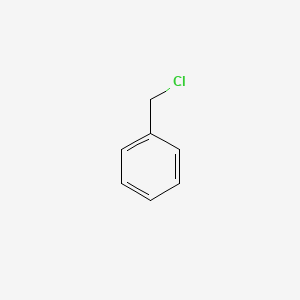 |
0.486 | D0R0UJ | 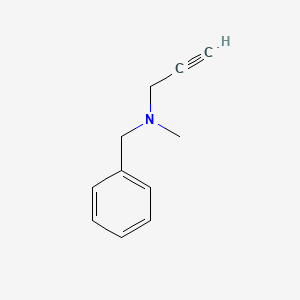 |
0.404 | ||
| ENC000203 |  |
0.486 | D0P6UB | 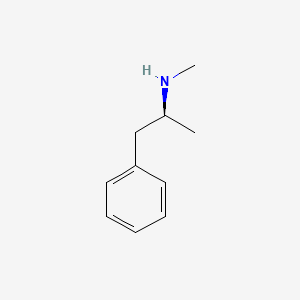 |
0.400 | ||
| ENC000014 | 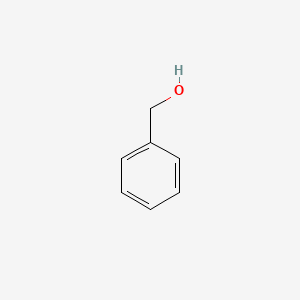 |
0.486 | D0P2GK | 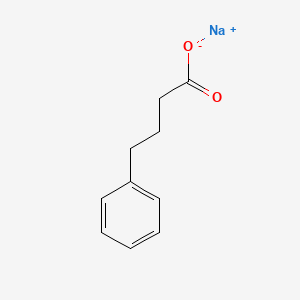 |
0.396 | ||
| ENC000215 | 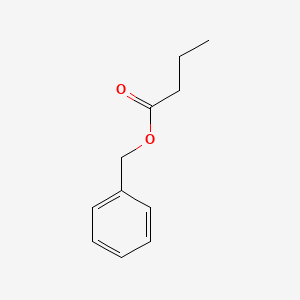 |
0.468 | D0G1OZ | 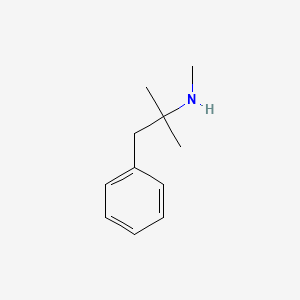 |
0.383 | ||
| ENC001728 | 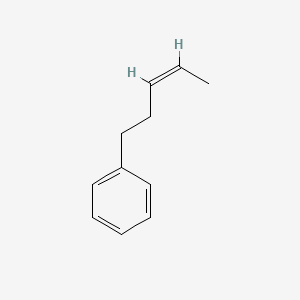 |
0.455 | D0R1CR |  |
0.383 | ||