NPs Basic Information
|
Name |
Benzyl Chloride
|
| Molecular Formula | C7H7Cl | |
| IUPAC Name* |
chloromethylbenzene
|
|
| SMILES |
C1=CC=C(C=C1)CCl
|
|
| InChI |
InChI=1S/C7H7Cl/c8-6-7-4-2-1-3-5-7/h1-5H,6H2
|
|
| InChIKey |
KCXMKQUNVWSEMD-UHFFFAOYSA-N
|
|
| Synonyms |
BENZYL CHLORIDE; (Chloromethyl)benzene; 100-44-7; Chloromethylbenzene; Benzylchloride; alpha-Chlorotoluene; Benzylchlorid; Tolyl chloride; Chlorophenylmethane; Chlorure de benzyle; alpha-Chlortoluol; Benzene, (chloromethyl)-; Benzene, chloromethyl-; a-Chlorotoluene; omega-Chlorotoluene; RCRA waste number P028; NCI-C06360; .alpha.-Chlorotoluene; Benzile (cloruro di); Benzyle (chlorure de); benzyl-chloride; Toluene, .alpha.-chloro-; NSC 8043; chloromethyl-benzene; .alpha.-Chlortoluol; Phenylmethyl chloride; .omega.-Chlorotoluene; CHEBI:615597; 83H19HW7K6; NSC-8043; Benzylchlorid [German]; Toluene, alpha-chloro-; alpha-Chlortoluol [German]; CCRIS 79; Chlorure de benzyle [French]; 1-chloromethylbenzene; HSDB 368; Toluene, ar-chloro-; Benzile (cloruro di) [Italian]; Benzyle (chlorure de) [French]; (Chloromethyl)-benzene; EINECS 202-853-6; UN1738; RCRA waste no. P028; benzyi chloride; benzyl choride; UNII-83H19HW7K6; AI3-15518; BnCl; alpha-chloro-toluene; Benzile(cloruro di); PhCH2Cl; Benzyle(chlorure de); 1-(chloromethyl)benzene; C6H5CH2Cl; DSSTox_CID_153; EC 202-853-6; TOLUENE,ALPHA-CHLORO; SCHEMBL7413; WLN: G1R; DSSTox_RID_75405; DSSTox_GSID_20153; BENZYL CHLORIDE [II]; BENZYL CHLORIDE [MI]; Benzyl chloride, unstabilized; Benzyl chloride, ReagentPlus,; BENZYL CHLORIDE [HSDB]; Benzene, (chloromethyl)- (VA; CHEMBL498878; Benzyl chloride, AR, >=99%; DTXSID0020153; BENZYL CHLORIDE [USP-RS]; NSC8043; ZINC1586371; Tox21_200266; MFCD00000889; AKOS000118784; UN 1738; Benzyl chloride [UN1738] [Poison]; NCGC00090818-01; NCGC00090818-02; NCGC00257820-01; CAS-100-44-7; Benzyl chloride, purum, >=99.0% (GC); DB-058434; B0412; Benzyl chloride, puriss., >=99.5% (GC); FT-0622815; FT-0662715; C19167; Q412260; Q-200697; BENZALKONIUM CHLORIDE IMPURITY C [EP IMPURITY]; Benzyl chloride, unstabilized [UN1738] [Poison, Corrosive]; Benzyl chloride, ReagentPlus(R), 99%, contains <=1% propylene oxide as stabilizer; 9CL
|
|
| CAS | 100-44-7 | |
| PubChem CID | 7503 | |
| ChEMBL ID | CHEMBL498878 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 126.58 | ALogp: | 2.3 |
| HBD: | 0 | HBA: | 0 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 0.0 | Aromatic Rings: | 1 |
| Heavy Atoms: | 8 | QED Weighted: | 0.507 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.304 | MDCK Permeability: | 0.00003590 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.003 |
| 30% Bioavailability (F30%): | 0.006 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.939 | Plasma Protein Binding (PPB): | 87.90% |
| Volume Distribution (VD): | 2.353 | Fu: | 10.50% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.956 | CYP1A2-substrate: | 0.849 |
| CYP2C19-inhibitor: | 0.8 | CYP2C19-substrate: | 0.31 |
| CYP2C9-inhibitor: | 0.248 | CYP2C9-substrate: | 0.328 |
| CYP2D6-inhibitor: | 0.055 | CYP2D6-substrate: | 0.326 |
| CYP3A4-inhibitor: | 0.025 | CYP3A4-substrate: | 0.332 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 10.364 | Half-life (T1/2): | 0.808 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.032 | Human Hepatotoxicity (H-HT): | 0.271 |
| Drug-inuced Liver Injury (DILI): | 0.529 | AMES Toxicity: | 0.232 |
| Rat Oral Acute Toxicity: | 0.08 | Maximum Recommended Daily Dose: | 0.034 |
| Skin Sensitization: | 0.91 | Carcinogencity: | 0.46 |
| Eye Corrosion: | 0.996 | Eye Irritation: | 0.996 |
| Respiratory Toxicity: | 0.971 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000203 |  |
0.643 | D05OIS |  |
0.643 | ||
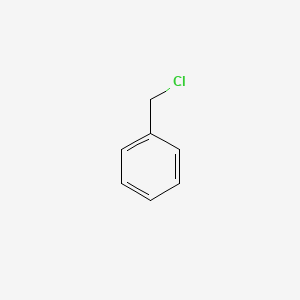
| ENC000014 | 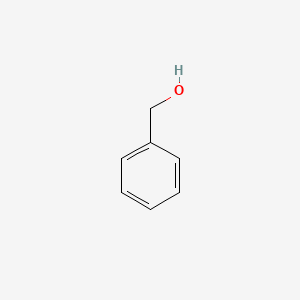 |
0.643 | D05BMG |  |
0.545 | ||
| ENC000217 | 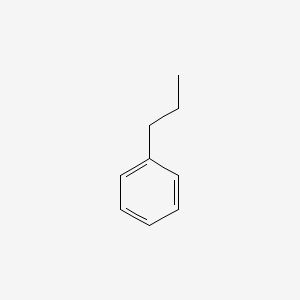 |
0.581 | D0T3LF | 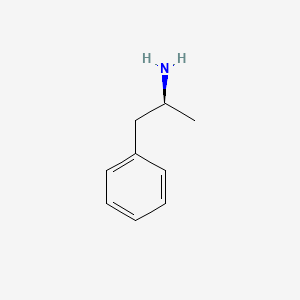 |
0.545 | ||
| ENC000128 | 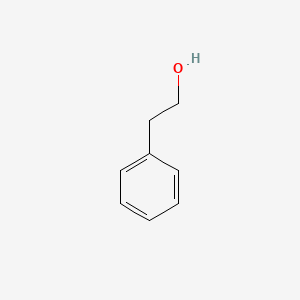 |
0.581 | D0P9AC | 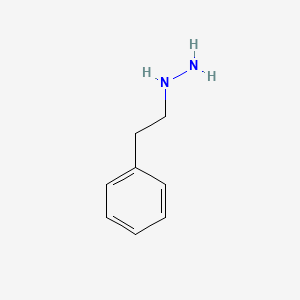 |
0.529 | ||
| ENC000053 | 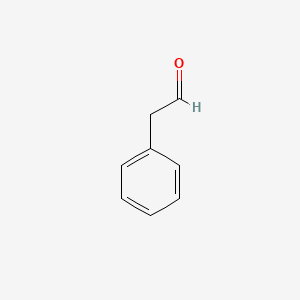 |
0.581 | D0U0RZ | 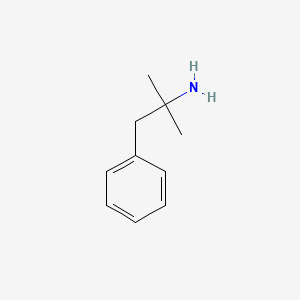 |
0.514 | ||
| ENC000219 | 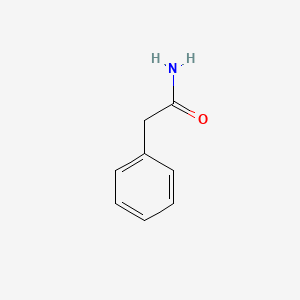 |
0.545 | D0P6UB | 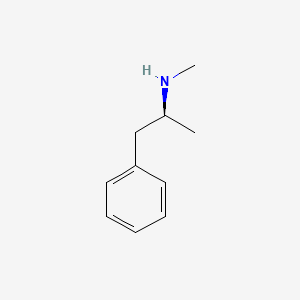 |
0.500 | ||
| ENC005854 |  |
0.545 | D00DZN |  |
0.500 | ||
| ENC000218 | 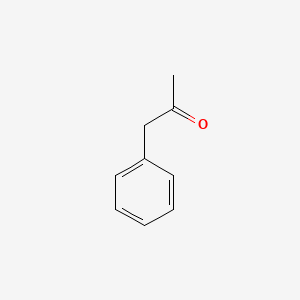 |
0.545 | D0G1OZ | 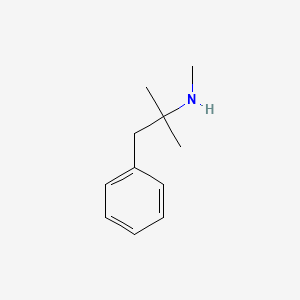 |
0.474 | ||
| ENC000054 | 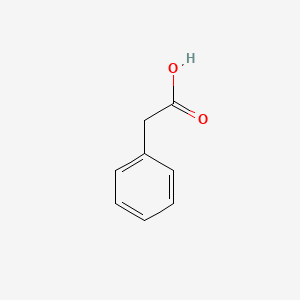 |
0.545 | D0R1CR |  |
0.474 | ||
| ENC000004 | 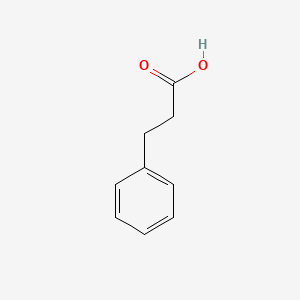 |
0.500 | D0R0UJ | 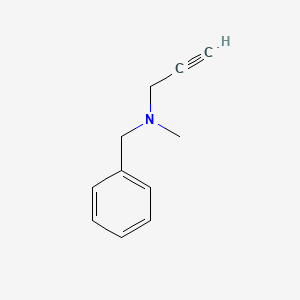 |
0.462 | ||