NPs Basic Information
|
Name |
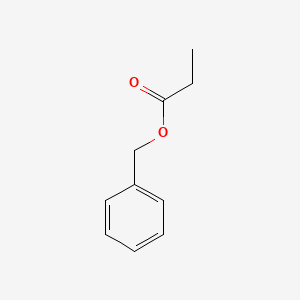
Benzyl propionate
|
| Molecular Formula | C10H12O2 | |
| IUPAC Name* |
benzyl propanoate
|
|
| SMILES |
CCC(=O)OCC1=CC=CC=C1
|
|
| InChI |
InChI=1S/C10H12O2/c1-2-10(11)12-8-9-6-4-3-5-7-9/h3-7H,2,8H2,1H3
|
|
| InChIKey |
VHOMAPWVLKRQAZ-UHFFFAOYSA-N
|
|
| Synonyms |
BENZYL PROPIONATE; 122-63-4; Benzyl propanoate; Propanoic acid, phenylmethyl ester; Propionic acid, benzyl ester; Propionic Acid Benzyl Ester; Phenylmethyl propanoate; Phenylmethyl propionate; Benzyl propionate (natrual); FEMA No. 2150; Benzyl n-propionate; Propionic acid-benzyl ester; NSC-46100; 307DN1208L; Benzyl propinate; EINECS 204-559-3; NSC 46100; benzylpropanoate; benzyl propanate; AI3-02952; UNII-307DN1208L; enzyl n-propionate; MFCD00027003; propionic acid benzyl; Nat.Benzyl Propionate; (phenylmethyl) propanoate; propanoic acid benzyl ester; EC 204-559-3; Propionic acid, benzyl ester (6CI,7CI,8CI); DSSTox_CID_24791; DSSTox_RID_80479; DSSTox_GSID_44791; SCHEMBL111605; BENZYL PROPIONATE [FCC]; BENZYL PROPIONATE [FHFI]; CHEMBL3185609; DTXSID4044791; FEMA 2150; CHEBI:180401; ZINC394884; Benzyl propionate, >=98%, FCC; NSC46100; propanoic acid (phenylmethyl) ester; Tox21_301783; Benzyl propionate, analytical standard; AKOS005206945; DS-6366; Benzyl propionate, >=98%, FCC, FG; NCGC00256011-01; AC-17034; CAS-122-63-4; TETRAMETHYLAMMONIUMHYDROGENPHTHALATE; CS-0017193; FT-0622781; P0501; Benzyl propionate, natural, >=98%, FCC, FG; E80882; A804933; Q10869281
|
|
| CAS | 122-63-4 | |
| PubChem CID | 31219 | |
| ChEMBL ID | CHEMBL3185609 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 164.2 | ALogp: | 2.4 |
| HBD: | 0 | HBA: | 2 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 26.3 | Aromatic Rings: | 1 |
| Heavy Atoms: | 12 | QED Weighted: | 0.642 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.288 | MDCK Permeability: | 0.00004950 |
| Pgp-inhibitor: | 0.002 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.003 |
| 30% Bioavailability (F30%): | 0.013 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.721 | Plasma Protein Binding (PPB): | 82.47% |
| Volume Distribution (VD): | 1.332 | Fu: | 17.35% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.971 | CYP1A2-substrate: | 0.178 |
| CYP2C19-inhibitor: | 0.859 | CYP2C19-substrate: | 0.238 |
| CYP2C9-inhibitor: | 0.195 | CYP2C9-substrate: | 0.149 |
| CYP2D6-inhibitor: | 0.035 | CYP2D6-substrate: | 0.18 |
| CYP3A4-inhibitor: | 0.023 | CYP3A4-substrate: | 0.377 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 13.337 | Half-life (T1/2): | 0.891 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.114 | Human Hepatotoxicity (H-HT): | 0.049 |
| Drug-inuced Liver Injury (DILI): | 0.615 | AMES Toxicity: | 0.065 |
| Rat Oral Acute Toxicity: | 0.035 | Maximum Recommended Daily Dose: | 0.015 |
| Skin Sensitization: | 0.535 | Carcinogencity: | 0.341 |
| Eye Corrosion: | 0.475 | Eye Irritation: | 0.959 |
| Respiratory Toxicity: | 0.038 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000215 | 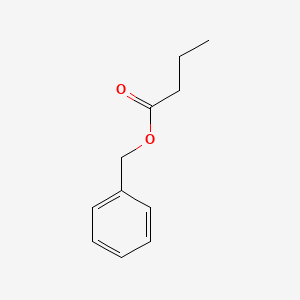 |
0.775 | D0G1VX |  |
0.473 | ||
| ENC000597 | 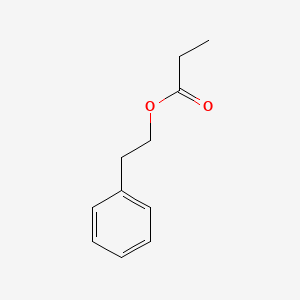 |
0.732 | D0P2GK |  |
0.468 | ||
| ENC000308 | 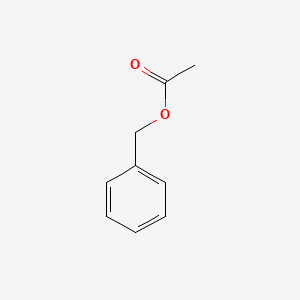 |
0.711 | D05OIS |  |
0.462 | ||
| ENC000214 | 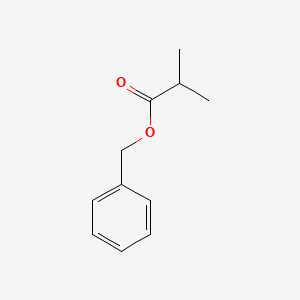 |
0.628 | D0R1CR |  |
0.457 | ||
| ENC000208 | 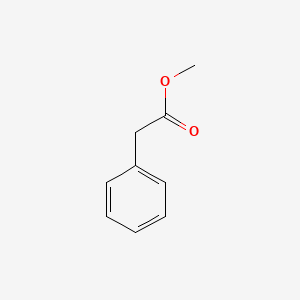 |
0.585 | D00DZN |  |
0.449 | ||
| ENC000216 | 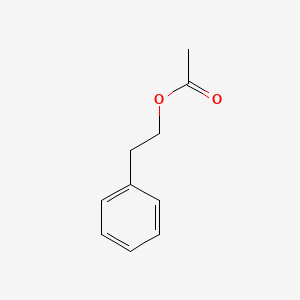 |
0.581 | D0T3LF | 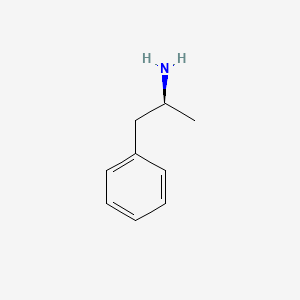 |
0.442 | ||
| ENC000779 | 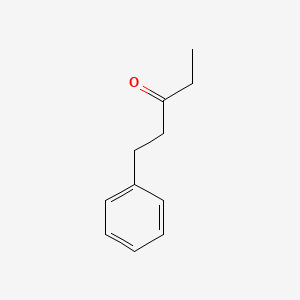 |
0.581 | D05BMG |  |
0.442 | ||
| ENC000218 | 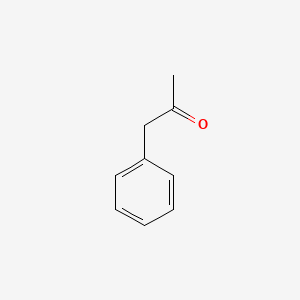 |
0.550 | D0P9AC | 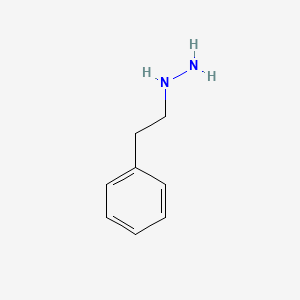 |
0.432 | ||
| ENC000598 | 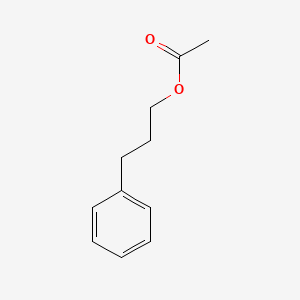 |
0.543 | D07ONP | 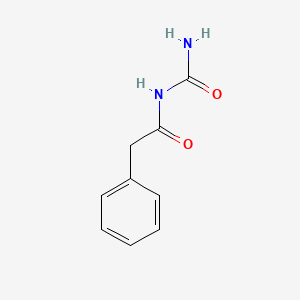 |
0.429 | ||
| ENC000203 |  |
0.541 | D0U0RZ | 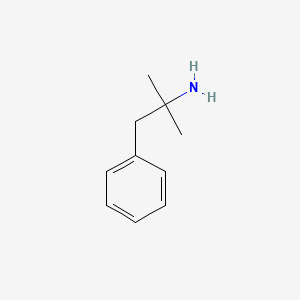 |
0.422 | ||