NPs Basic Information
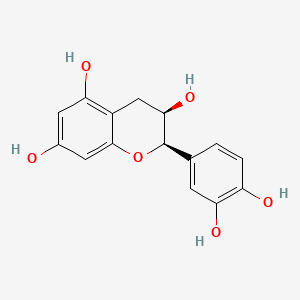
|
Name |
(-)-Epicatechin
|
| Molecular Formula | C15H14O6 | |
| IUPAC Name* |
(2R,3R)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol
|
|
| SMILES |
C1[C@H]([C@H](OC2=CC(=CC(=C21)O)O)C3=CC(=C(C=C3)O)O)O
|
|
| InChI |
InChI=1S/C15H14O6/c16-8-4-11(18)9-6-13(20)15(21-14(9)5-8)7-1-2-10(17)12(19)3-7/h1-5,13,15-20H,6H2/t13-,15-/m1/s1
|
|
| InChIKey |
PFTAWBLQPZVEMU-UKRRQHHQSA-N
|
|
| Synonyms |
(-)-Epicatechin; Epicatechin; 490-46-0; L-Epicatechin; (-)-Epicatechol; Epicatechol; l-Acacatechin; (2R,3R)-2-(3,4-Dihydroxyphenyl)chroman-3,5,7-triol; epi-Catechin; epi-Catechol; (-)epicatechin; (2R,3R)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol; l-Epicatechol; (-)-epi catechin; (2R,3R)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-2H-1-benzopyran-3,5,7-triol; CHEBI:90; Epicatechol, (-)-; (2R,3R)-(-)-Epicatechin; (-)-(2R:3R)-5,7,3',4'-Tetrahydroxyflavan-3-ol; 34PHS7TU43; NSC81161; 2H-1-Benzopyran-3,5,7-triol, 2-(3,4-dihydroxyphenyl)-3,4-dihydro-, (2R,3R)-; NSC-81161; (-)-cis-Epicatechin; NSC 81161; 2H-1-Benzopyran-3,5,7-triol, 2-(3,4-dihydroxyphenyl)-3,4-dihydro-, (2R-cis)-; DSSTox_CID_25133; DSSTox_RID_80694; DSSTox_GSID_45133; (2R,3R)-2-(3,4-dihydroxyphenyl)chromane-3,5,7-triol; Epicatechin-(-); CAS-490-46-0; SMR000156230; CCRIS 7097; EINECS 207-710-1; UNII-34PHS7TU43; Acacatechin; Colatein; alpha-catechin; Kakaol; Teacatechin I; .alpha. Catechin; NCGC00015215-02; 28E; 7295-85-4; L(-)-Epicatechin; Prestwick_203; 2,3-cis-epicatechin; L-Epicatechin ,(S); Spectrum_000159; SpecPlus_000267; 2-(3,4-Dihydroxyphenyl)-2,3,4-trihydro-3,5,7-trihydroxychromene; Spectrum2_000675; Spectrum3_000243; Spectrum4_000949; Spectrum5_000929; Lopac-C-1251; Oprea1_209947; SCHEMBL19412; BSPBio_001626; KBioGR_001538; KBioSS_000639; SPECTRUM210206; cid_72276; MLS001304012; MLS001304152; DivK1c_006363; SPBio_000769; CHEMBL583912; L-EPICATECHIN [WHO-DD]; DTXSID4045133; ACon1_001106; BCBcMAP01_000224; BDBM23417; KBio1_001307; KBio2_000639; KBio2_003207; KBio2_005775; KBio3_001126; HMS1923M05; KUC104404N; ZINC119988; (-)-EPICATECHIN [USP-RS]; HY-N0001; Tox21_110101; 3,3',4',5,7-Pentahydroxyflavane; CCG-38571; LMPK12020003; MFCD00075648; s4723; (-)-Epicatechin, analytical standard; AKOS015895981; Tox21_110101_1; (-)-Epicatechin, >=90% (HPLC); CS-3760; DB12039; DS-3358; KSC-10-144; SDCCGMLS-0066927.P001; 2H-1-Benzopyran-3,5,7-triol, 2-(3,4-dihydroxyphenyl)-3,4-dihydro-,(2R,3R)-; SMP1_000115; NCGC00015215-01; NCGC00016415-01; NCGC00016415-02; NCGC00016415-03; NCGC00016415-04; NCGC00017331-08; AC-14586; BP-30203; CAS-154-23-4; CAS-7295-85-4; E1226; (-)-cis-3,3',4',5,7-Pentahydroxyflavane; (-)-Epicatechin 1000 microg/mL in Acetone; A12043; C09727; O10053; (-)-Epicatechin 1000 microg/mL in Acetonitrile; 490E460; A871843; (-)-Epicatechin, >=98% (HPLC), from green tea; Q-200001; CF3BA0C2-DE1B-44AB-A4D3-800F017221BA; Epicatechin, primary pharmaceutical reference standard; Q23050136; (-)-EPICATECHIN (CONSTITUENT OF MARITIME PINE) [DSC]; 2-(3,4-dihydroxyphenyl)-2,3,4-trihydro-3,5,7-trihydroxychromene;L-Epicatechin; (-)-cis-3,3',4',5,7-Pentahydroxyflavane, (2R,3R)-2-(3,4-Dihydroxyphenyl)-3,4-dihydro-1(2H)-benzopyran-3,5,7-triol
|
|
| CAS | 17334-50-8 | |
| PubChem CID | 72276 | |
| ChEMBL ID | CHEMBL583912 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 290.27 | ALogp: | 0.4 |
| HBD: | 5 | HBA: | 6 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 110.0 | Aromatic Rings: | 3 |
| Heavy Atoms: | 21 | QED Weighted: | 0.514 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -6.213 | MDCK Permeability: | 0.00000412 |
| Pgp-inhibitor: | 0.007 | Pgp-substrate: | 0.004 |
| Human Intestinal Absorption (HIA): | 0.037 | 20% Bioavailability (F20%): | 0.998 |
| 30% Bioavailability (F30%): | 0.999 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.025 | Plasma Protein Binding (PPB): | 92.36% |
| Volume Distribution (VD): | 0.652 | Fu: | 8.35% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.393 | CYP1A2-substrate: | 0.224 |
| CYP2C19-inhibitor: | 0.031 | CYP2C19-substrate: | 0.054 |
| CYP2C9-inhibitor: | 0.323 | CYP2C9-substrate: | 0.827 |
| CYP2D6-inhibitor: | 0.139 | CYP2D6-substrate: | 0.31 |
| CYP3A4-inhibitor: | 0.371 | CYP3A4-substrate: | 0.18 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 16.512 | Half-life (T1/2): | 0.884 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.03 | Human Hepatotoxicity (H-HT): | 0.099 |
| Drug-inuced Liver Injury (DILI): | 0.101 | AMES Toxicity: | 0.616 |
| Rat Oral Acute Toxicity: | 0.43 | Maximum Recommended Daily Dose: | 0.146 |
| Skin Sensitization: | 0.947 | Carcinogencity: | 0.159 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.914 |
| Respiratory Toxicity: | 0.117 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000320 | 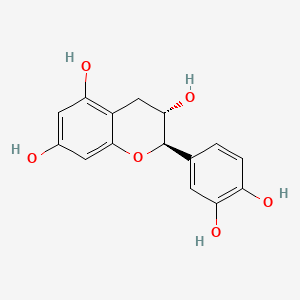 |
1.000 | D07MGA |  |
0.469 | ||
| ENC001068 | 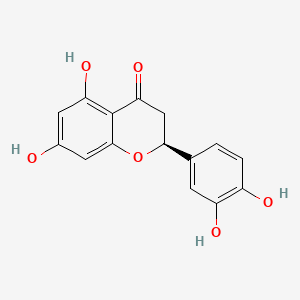 |
0.611 | D0K8KX |  |
0.439 | ||
| ENC000700 | 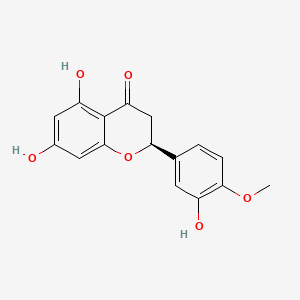 |
0.469 | D04AIT |  |
0.432 | ||
| ENC001438 |  |
0.443 | D0AZ8C |  |
0.381 | ||
| ENC001529 |  |
0.439 | D0U3YB |  |
0.326 | ||
| ENC001534 | 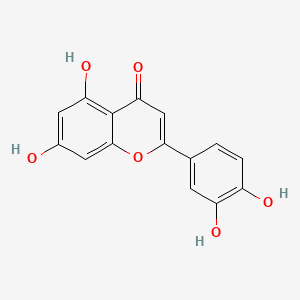 |
0.432 | D0R6BI | 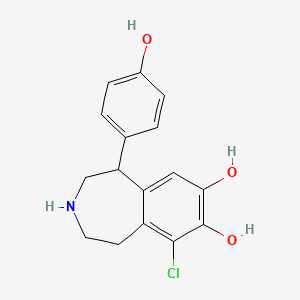 |
0.315 | ||
| ENC004203 | 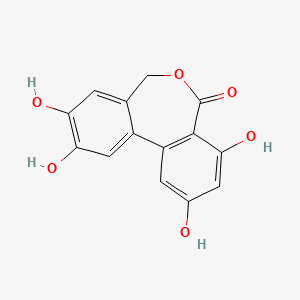 |
0.378 | D02FCQ | 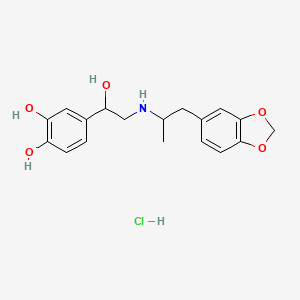 |
0.283 | ||
| ENC004389 |  |
0.375 | D0KN2M |  |
0.282 | ||
| ENC003305 |  |
0.368 | D06KYN |  |
0.268 | ||
| ENC000940 | 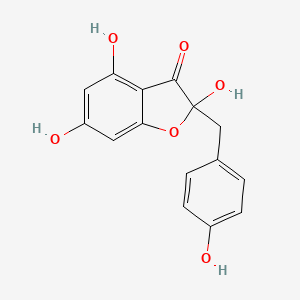 |
0.349 | D07MOX | 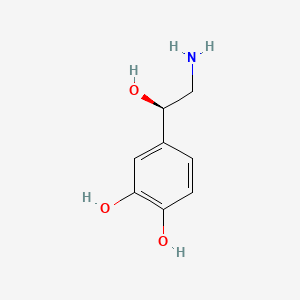 |
0.268 | ||