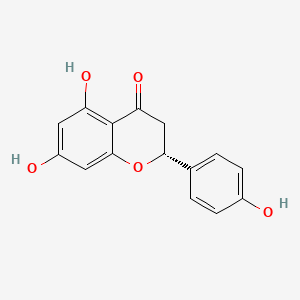
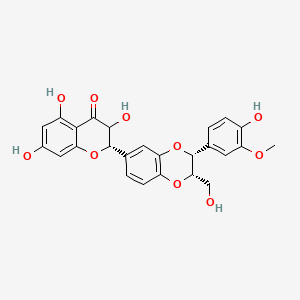
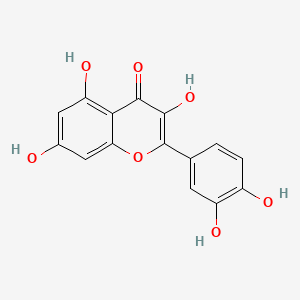
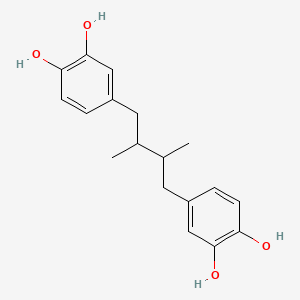
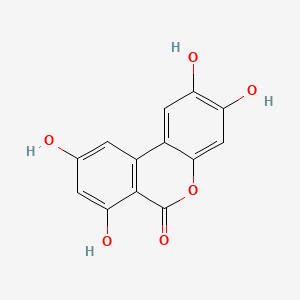
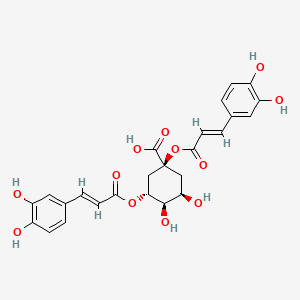
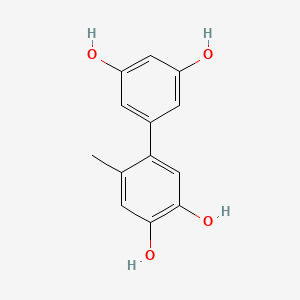
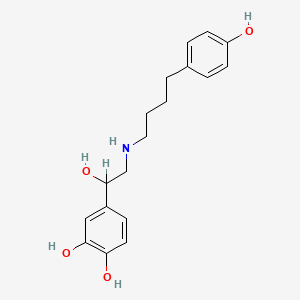
| Synonyms |
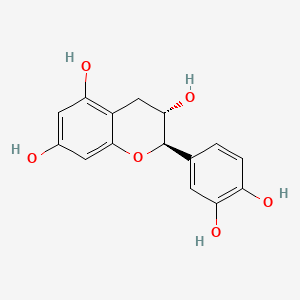
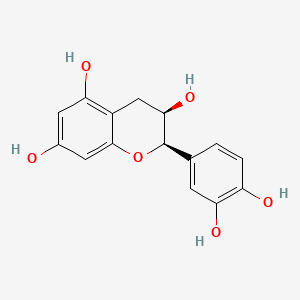
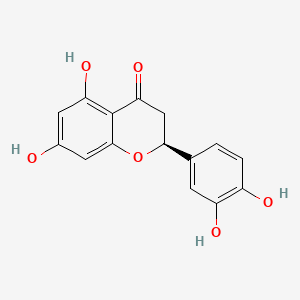
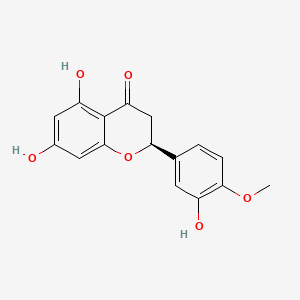
(+)-catechin; Cianidanol; CATECHIN; 154-23-4; Catechuic acid; Catechinic acid; Cyanidanol; D-Catechin; Catergen; Cianidol; (+)-Catechol; (+)-Cyanidanol; Biocatechin; (+)-Cyanidan-3-ol; D-Catechol; D-(+)-Catechin; Dexcyanidanol; (+)-Catechin Hydrate; Catechin (flavan); Catechol (flavan); (2R,3S)-Catechin; (2R,3S)-2-(3,4-Dihydroxyphenyl)chroman-3,5,7-triol; 3-Cyanidanol, (+)-; DL-Catechin; (2R,3S)-(+)-Catechin; Cianidanolum; dl-Catechol; (+)-Cianidanol; 3,3',4',5,7-Flavanpentol; KB-53; (+)-Cyanidanol-3; 7295-85-4; CCRIS 6855; (+)-3',4',5,7-Tetrahydroxy-2,3-trans-flavan-3-ol; (2R,3S)-2-(3,4-dihydroxyphenyl)chromane-3,5,7-triol; (2R,3S)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol; 2H-1-Benzopyran-3,5,7-triol, 2-(3,4-dihydroxyphenyl)-3,4-dihydro-, (2R-trans)-; NSC 2819; NSC-2819; Catechin, d-; Catechol (+); CHEBI:15600; AI3-22757; NSC2819; (+)-(2R,3S)-5,7,3',4'-Tetrahydroxyflavan-3-ol; CATECHIN, D; (2R,3S)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-2H-1-benzopyran-3,5,7-triol; Catechin-(+,-) hydrate; 2H-1-benzopyran-3,5,7-triol, 2-(3,4-dihydroxyphenyl)-3,4-dihydro-, (2R,3S)-; 8R1V1STN48; CHEMBL311498; (2R-trans)-2-(3,4-Dihydroxyphenyl)-3,4-dihydro-2H-1-benzopyran-3,5,7-triol; 5J4Y243W61; 100786-01-4; 225937-10-0; Catechu; Gambier; Transepar; Katha; trans-(+)-3,3',4',5,7-Flavanpentol; Zyma; Cyanidanol-3; (+)-Cyanidol-3; Cutch (dye); (+)-(2R:3S)-5,7,3',4'-Tetrahydroxyflavan-3-ol; Epicatechin-(-); Cianidanol [INN:JAN]; MLS001056745; Cianidanolum [INN-Latin]; Catechine dl-form; (+-)-catechin; 2,3-trans-catechin; 2,3-Dihydro-4-desoxoquercetin; KB 53; SR-01000075742; SMR000326724; EINECS 205-825-1; UNII-8R1V1STN48; Catechinate; Catechuate; Drenoliver; rac-catechin; (2R,3S)-3,3',4',5,7-Flavanpentol; Tanningenic acid; DL-Catechine; UNII-5J4Y243W61; Z 7300; Teafuran 30A; KXN; Prestwick_998; Sunkatol No. 1; EINECS 230-731-2; (+/-)-catechol; 2-(3,4-Dihydroxyphenyl)chromane-3,5,7-triol; RACEMIC CATECHIN; Spectrum_000395; (+)-Catechin,(S); 2-(3,4-Dihydroxyphenyl)-3,5,7-chromanetriol #; CATECHIN [MI]; CATECHIN, DL-; CATECHIN [VANDF]; CIANIDANOL [INN]; CIANIDANOL [JAN]; Prestwick0_000642; Prestwick0_000817; Prestwick1_000642; Prestwick1_000817; Prestwick2_000642; Prestwick2_000817; Prestwick3_000642; Spectrum2_000167; Spectrum3_000242; Spectrum4_001763; Spectrum5_000345; Epitope ID:116872; trans-2-(3,4-Dihydroxyphenyl)-3,4-dihydro-2H-1-benzopyran-3,5,7-triol; CIANIDANOL [MART.]; CIANIDANOL [WHO-DD]; Lopac0_000219; SCHEMBL19741; BSPBio_000643; BSPBio_001624; KBioGR_002245; KBioSS_000875; BIDD:ER0378; DivK1c_000647; (+)-2-(3,4-Dihydroxyphenyl)-3,5,7-chromantriol; SPBio_000033; SPBio_002564; SPBio_002634; CATECHIN DL-FORM [MI]; BPBio1_000709; cid_107957; (3S,2R)-2-(3,4-dihydroxyphenyl)chromane-3,5,7-triol; CATECHOL, (+/-)-; DTXSID3022322; ACon1_001489; BDBM23416; BDBM60836; CIANIDANOL, (+/-)-; HMS502A09; KBio1_000647; KBio2_000875; KBio2_003443; KBio2_006011; KBio3_001124; YK-85 Light Yellow Powder 85; (+)-CATECHIN [USP-RS]; 4c94; NINDS_000647; DTXSID001349029; HMS1570A05; HMS1570D15; HMS2097A05; HMS3260L19; Pharmakon1600-00210205; ZINC119983; (+)-Catechin, analytical standard; HY-N0898; TNP00270; Tox21_500219; CCG-40007; LMPK12020001; NSC755824; s3974; s4722; STL570276; trans3,3,4,5,7 pentahydroxyflavane; AKOS015960546; CS-3759; DB14086; LP00219; ND-0342; NSC-755824; SDCCGMLS-0066526.P001; SDCCGSBI-0050207.P004; IDI1_000647; NCGC00017331-01; NCGC00017331-02; NCGC00017331-03; NCGC00017331-04; NCGC00017331-05; NCGC00017331-19; NCGC00093689-01; NCGC00093689-02; NCGC00093689-03; NCGC00260904-01; AC-11608; AC-35859; AS-72772; NCI60_002303; (+)-Catechin 1000 microg/mL in Acetone; SBI-0050207.P003; EU-0100219; ( inverted exclamation markA)-Catechin hydrate; (+)-Catechin 1000 microg/mL in Acetonitrile; C 1251; C06562; D95105; H10916; AB00051886_13; EN300-6498629; (+/-)-Catechin 1000 microg/mL in Acetonitrile; 154C234; A809512; A878497; NATURAL BROWN 3 (CUTCH EXTRA OR GAMBIER); Q415007; Q-100183; SR-01000075742-1; SR-01000075742-7; SR-01000075742-8; SR-01000075742-9; BRD-K58736316-001-07-9; BRD-K58736316-001-08-7; SR-01000075742-10; SR-01000075742-12; SR-01000075742-14; (+)-CATECHIN (CONSTITUENT OF MARITIME PINE) [DSC]; D4A04A57-7609-451F-A446-53F4DFAD15F5; (2R,3S)-2-(3,4-dihydroxyphenyl)chroman-3,5,7-triol;hydrate; (+)-Catechin, United States Pharmacopeia (USP) Reference Standard; (2R,3S)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-1H-chromene-3,5,7-triol; (+)-Catechin, Pharmaceutical Secondary Standard; Certified Reference Material; (2R,3S)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-2H-1-benzopyran-3,5,7-triol;hydrate; (2R,3S)-2-[3,4-bis(oxidanyl)phenyl]-3,4-dihydro-2H-chromene-3,5,7-triol;hydrate; (2R-trans)-2-(3,4-Dihydroxyphenyl)-3-4-dihydro-2H-1-benzopyran-3,5,7-triol; 2-(3,4-Dihydroxyphenyl)-3,4-dihydro-2H-1-benzopyran-3,5,7-triol, (2R-trans); 2H-1-Benzopyran-3,7-triol, 2-(3,4-dihydroxyphenyl)-3,4-dihydro-, (2R-trans)-; 2H-1-BENZOPYRAN-3,5,7-TRIOL, 2-(3,4-DIHYDROXYPHENYL)-3,4-DIHYDRO-, (2S,3R)-REL-; 321-01-7
|