NPs Basic Information
|
Name |
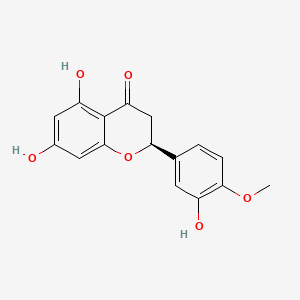
Hesperetin
|
| Molecular Formula | C16H14O6 | |
| IUPAC Name* |
(2S)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-2,3-dihydrochromen-4-one
|
|
| SMILES |
COC1=C(C=C(C=C1)[C@@H]2CC(=O)C3=C(C=C(C=C3O2)O)O)O
|
|
| InChI |
InChI=1S/C16H14O6/c1-21-13-3-2-8(4-10(13)18)14-7-12(20)16-11(19)5-9(17)6-15(16)22-14/h2-6,14,17-19H,7H2,1H3/t14-/m0/s1
|
|
| InChIKey |
AIONOLUJZLIMTK-AWEZNQCLSA-N
|
|
| Synonyms |
hesperetin; 520-33-2; Hesperitin; 3',5,7-Trihydroxy-4'-methoxyflavanone; (-)-hesperetin; (S)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)chroman-4-one; YSO2; 5,7,3'-Trihydroxy-4'-methoxyflavanone; Cyanidanon 4'-methyl ether 1626; 41001-90-5; NSC 57654; Prestwick_908; (2S)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-2,3-dihydrochromen-4-one; (-)-(S)-hesperetin; NSC-57654; 4H-1-Benzopyran-4-one, 2,3-dihydro-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-, (S)-; Eriodictyol 4'-monomethyl ether; CHEBI:28230; Q9Q3D557F1; (2S)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-2,3-dihydro-4H-chromen-4-one; (2S)-hesperetin; (2S)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)chroman-4-one; (S)-2,3-dihydro-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-1-benzopyran-4-one; (+-)-Hesperetin; MFCD00075646; (+/-)-Hesperetin; (2s)-5,7-Dihydroxy-2-(3-Hydroxy-4-Methoxyphenyl)-2,3-Dihydro-4h-1-Benzopyran-4-One; EINECS 208-290-2; Hesperitine; UNII-Q9Q3D557F1; 4H-1-Benzopyran-4-one, 2,3-dihydro-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-, (2S)-; (2S)-5,7-Dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-2,3-dihydro-4H-1-benzopyran-4-one (Hesperetin); 6JP; TNP00238; (S)-2,3-Dihydro-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4-benzopyrone; 4'-Methoxy-3',5,7-trihydroxyflavanone; Spectrum_000181; HESPERETIN [MI]; Prestwick0_000124; Prestwick1_000124; Prestwick2_000124; Prestwick3_000124; Spectrum2_001793; Spectrum3_001104; Spectrum4_001935; Spectrum5_000683; HESPERETIN [FHFI]; HESPERETIN [INCI]; 5,7-Dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4-chromanone; Oprea1_828704; SCHEMBL39833; BSPBio_000168; BSPBio_002808; KBioGR_002311; KBioSS_000661; SPECTRUM310012; Flavanone, 3',5,7-trihydroxy-4'-methoxy- (VAN); MLS002154205; BIDD:ER0512; DivK1c_001039; SPBio_001745; SPBio_002107; BPBio1_000186; CHEMBL399121; DTXSID4022319; FEMA NO. 4313; BCBcMAP01_000087; BDBM23418; GTPL10953; HMS503O19; KBio1_001039; KBio2_000661; KBio2_003229; KBio2_005797; KBio3_002028; ZINC39092; NINDS_001039; HMS1568I10; HMS2095I10; HMS2230M09; HMS3649H22; HMS3884N11; NP-13; (2S)-5,7-dihydroxy-2-(3-hydroxy-4-methoxy-phenyl)chroman-4-one; BCP28273; HY-N0168; BBL104011; CCG-38441; LMPK12140003; s2308; STL557824; AKOS016339567; AC-7970; DB01094; KS-5307; SDCCGMLS-0066605.P001; IDI1_001039; SMP1_000148; NCGC00016482-01; NCGC00016482-02; NCGC00016482-03; NCGC00016482-04; NCGC00142415-01; NCGC00142415-02; 5,7, 3'-Trihydroxy-4'-methoxyflavanone; CAS-520-33-2; SMR001233491; Flavanone, 3',5,7-trihydroxy-4'-methoxy-; H0721; Hesperitin; Hesperin; YSO2; Prestwick_908; SW197026-2; C01709; H10029; A828900; Discontinued. Please see H289480 or H289501; Q411310; SR-01000946723; SR-01000946723-1; BRD-K30553453-001-05-8; BRD-K30553453-001-08-2; Flavanone, 3',5, 7-trihydroxy-4'-methoxy- (VAN); Flavanone, 3',5,7-trihydroxy-4'-methoxy- (VAN) (8CI); (S)-5,7-Dihydroxy-2-(3-hydroxy-4-methoxyphenyl)chroman-4-one;Hesperetin; (2S)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-3,4-dihydro-2H-1-benzopyran-4-one; 2,3-DIHYDRO-5,7-DIHYDROXY-2-(3-HYDROXY-4-METHOXYPHENYL)-4H-1-BENZOPYRAN-4-ONE; 4H-1-Benzopyran-4-one,2,3-dihydro-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-, (2S)-
|
|
| CAS | 520-33-2 | |
| PubChem CID | 72281 | |
| ChEMBL ID | CHEMBL399121 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 302.28 | ALogp: | 2.4 |
| HBD: | 3 | HBA: | 6 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 96.2 | Aromatic Rings: | 3 |
| Heavy Atoms: | 22 | QED Weighted: | 0.787 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.802 | MDCK Permeability: | 0.00001270 |
| Pgp-inhibitor: | 0.009 | Pgp-substrate: | 0.033 |
| Human Intestinal Absorption (HIA): | 0.007 | 20% Bioavailability (F20%): | 0.009 |
| 30% Bioavailability (F30%): | 0.974 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.042 | Plasma Protein Binding (PPB): | 93.58% |
| Volume Distribution (VD): | 0.808 | Fu: | 8.76% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.917 | CYP1A2-substrate: | 0.868 |
| CYP2C19-inhibitor: | 0.663 | CYP2C19-substrate: | 0.062 |
| CYP2C9-inhibitor: | 0.758 | CYP2C9-substrate: | 0.923 |
| CYP2D6-inhibitor: | 0.623 | CYP2D6-substrate: | 0.707 |
| CYP3A4-inhibitor: | 0.816 | CYP3A4-substrate: | 0.193 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 16.654 | Half-life (T1/2): | 0.764 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.059 | Human Hepatotoxicity (H-HT): | 0.179 |
| Drug-inuced Liver Injury (DILI): | 0.944 | AMES Toxicity: | 0.133 |
| Rat Oral Acute Toxicity: | 0.823 | Maximum Recommended Daily Dose: | 0.607 |
| Skin Sensitization: | 0.914 | Carcinogencity: | 0.522 |
| Eye Corrosion: | 0.011 | Eye Irritation: | 0.93 |
| Respiratory Toxicity: | 0.927 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001068 | 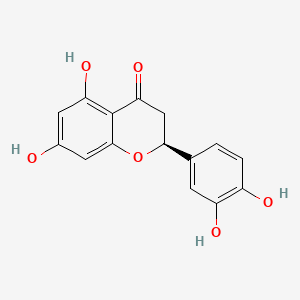 |
0.776 | D07MGA |  |
1.000 | ||
| ENC001438 | 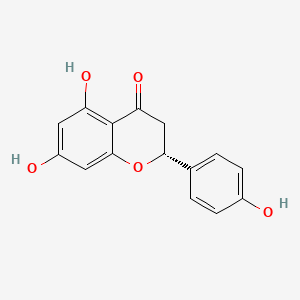 |
0.671 | D0I9HF | 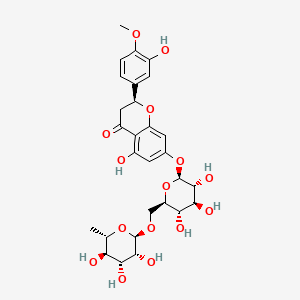 |
0.455 | ||
| ENC005245 | 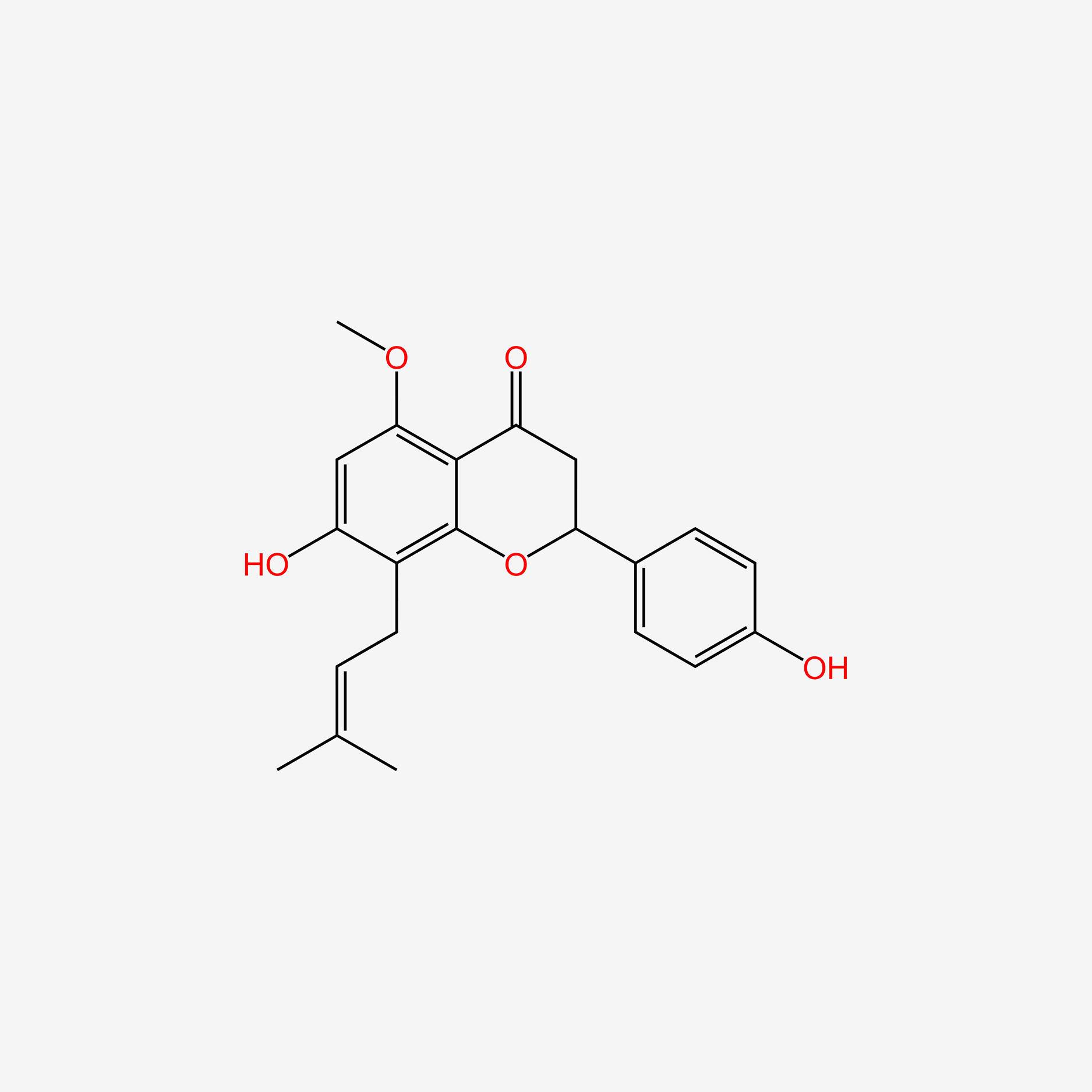 |
0.478 | D0AZ8C | 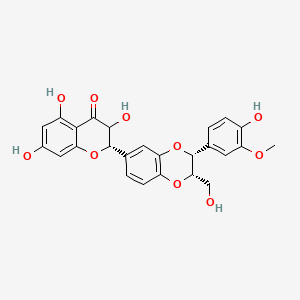 |
0.420 | ||
| ENC000699 | 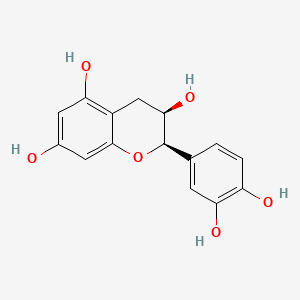 |
0.469 | D04AIT |  |
0.368 | ||
| ENC000320 | 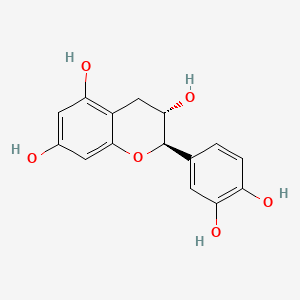 |
0.469 | D0K8KX |  |
0.360 | ||
| ENC005361 | 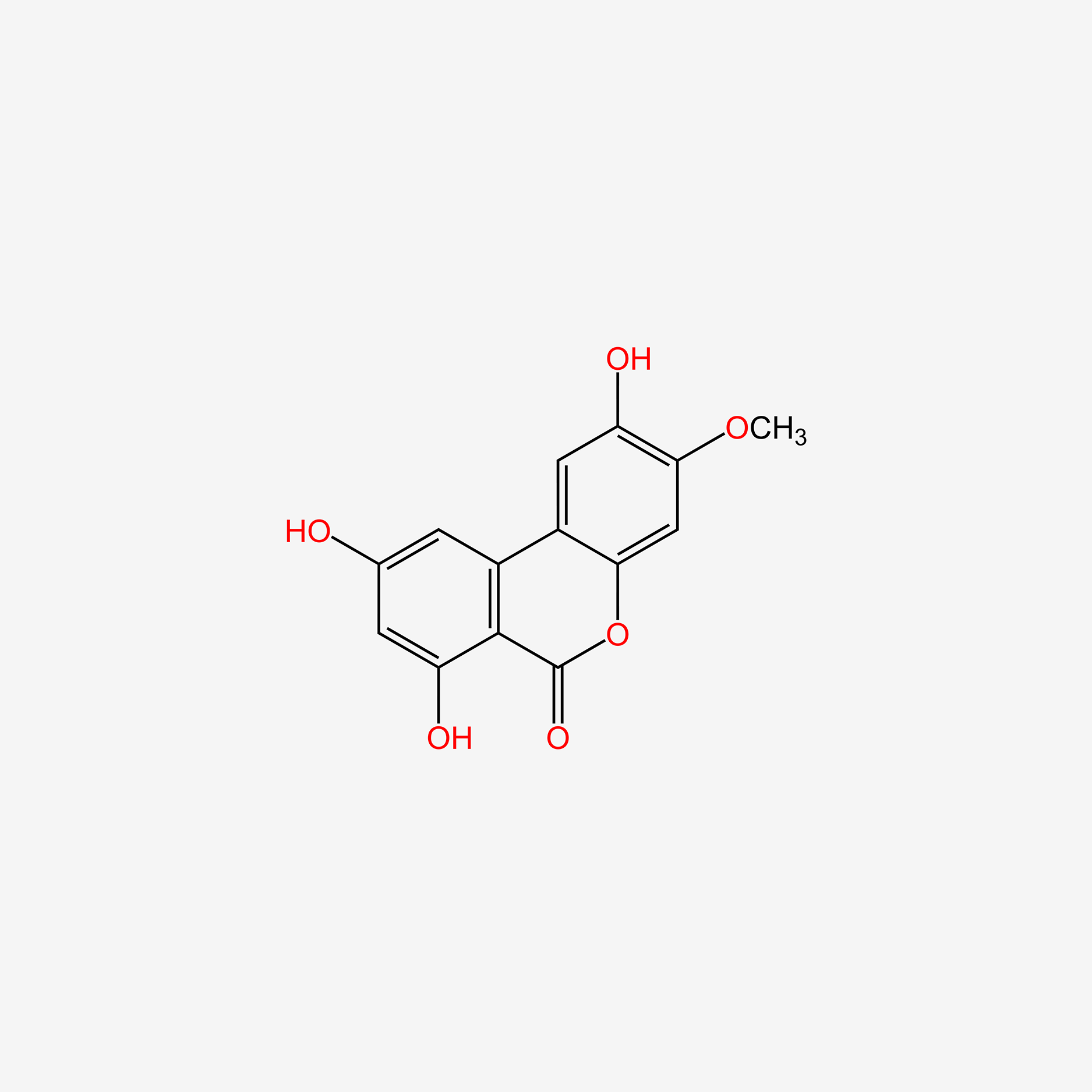 |
0.450 | D06GCK |  |
0.340 | ||
| ENC001773 |  |
0.450 | D0E9CD |  |
0.319 | ||
| ENC001085 | 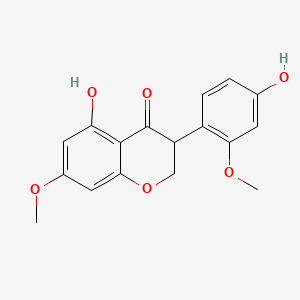 |
0.437 | D0R6BI | 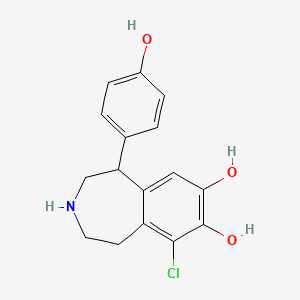 |
0.290 | ||
| ENC002587 | 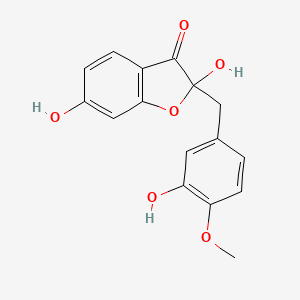 |
0.435 | D0L1JW |  |
0.282 | ||
| ENC005777 | 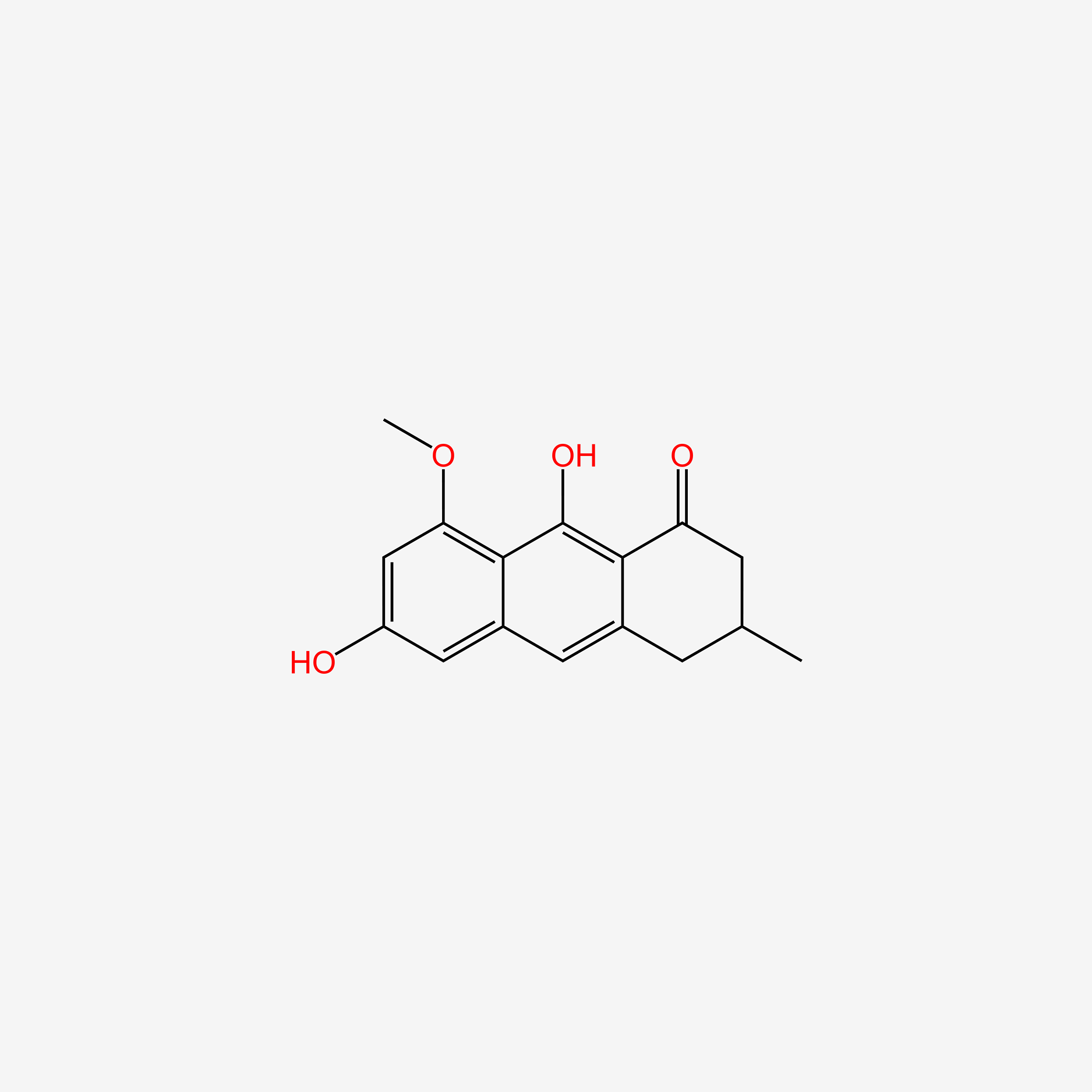 |
0.415 | D0F7CS | 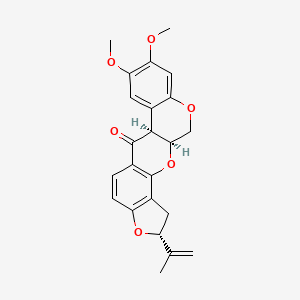 |
0.265 | ||