NPs Basic Information
|
Name |
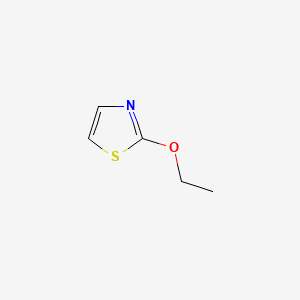
2-Ethoxythiazole
|
| Molecular Formula | C5H7NOS | |
| IUPAC Name* |
2-ethoxy-1,3-thiazole
|
|
| SMILES |
CCOC1=NC=CS1
|
|
| InChI |
InChI=1S/C5H7NOS/c1-2-7-5-6-3-4-8-5/h3-4H,2H2,1H3
|
|
| InChIKey |
NDUWJHRKDYXRAD-UHFFFAOYSA-N
|
|
| Synonyms |
2-Ethoxythiazole; 15679-19-3; 2-Ethoxy-1,3-thiazole; Thiazole, ethoxy-; Ethyl 2-thiazolyl ether; Thiazole, 2-ethoxy-; 2-Ethoxy thiazole; 2-Thiazolyl ethyl ether; 2-ethoxy-thiazole; FEMA No. 3340; Q3O421Q24K; UNII-Q3O421Q24K; EINECS 239-760-5; SCHEMBL577582; 2-ETHOXYTHIAZOLE [FHFI]; FEMA 3340; DTXSID00166130; 2-Ethoxythiazole, >=99%, FG; ZINC1850626; MFCD00055026; AKOS006341293; CS-W013447; BP-10158; BS-15721; DB-021032; E0669; FT-0637262; D84242; EN300-1196155; 679E193; A809774; Q-100187; Q27286962
|
|
| CAS | 15679-19-3 | |
| PubChem CID | 61809 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 129.18 | ALogp: | 1.6 |
| HBD: | 0 | HBA: | 3 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 50.4 | Aromatic Rings: | 1 |
| Heavy Atoms: | 8 | QED Weighted: | 0.61 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.055 | MDCK Permeability: | 0.00005760 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.002 | 20% Bioavailability (F20%): | 0.006 |
| 30% Bioavailability (F30%): | 0.718 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.864 | Plasma Protein Binding (PPB): | 69.45% |
| Volume Distribution (VD): | 1.465 | Fu: | 38.36% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.974 | CYP1A2-substrate: | 0.839 |
| CYP2C19-inhibitor: | 0.83 | CYP2C19-substrate: | 0.79 |
| CYP2C9-inhibitor: | 0.085 | CYP2C9-substrate: | 0.643 |
| CYP2D6-inhibitor: | 0.044 | CYP2D6-substrate: | 0.705 |
| CYP3A4-inhibitor: | 0.039 | CYP3A4-substrate: | 0.456 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 6.643 | Half-life (T1/2): | 0.405 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.01 | Human Hepatotoxicity (H-HT): | 0.062 |
| Drug-inuced Liver Injury (DILI): | 0.855 | AMES Toxicity: | 0.091 |
| Rat Oral Acute Toxicity: | 0.186 | Maximum Recommended Daily Dose: | 0.017 |
| Skin Sensitization: | 0.43 | Carcinogencity: | 0.391 |
| Eye Corrosion: | 0.786 | Eye Irritation: | 0.99 |
| Respiratory Toxicity: | 0.705 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001141 | 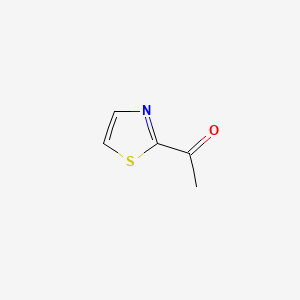 |
0.364 | D07INV |  |
0.218 | ||
| ENC001365 |  |
0.237 | D0P0HB |  |
0.205 | ||
| ENC000577 |  |
0.231 | D0L7UQ |  |
0.191 | ||
| ENC000650 |  |
0.205 | D02CKX | 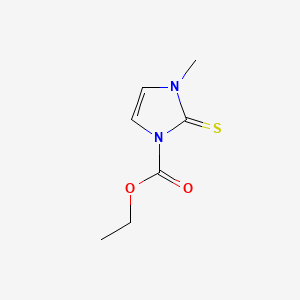 |
0.170 | ||
| ENC000106 | 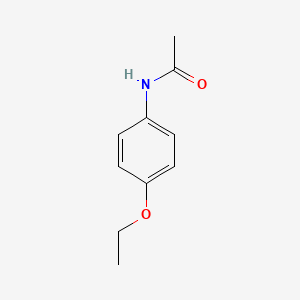 |
0.204 | D0Q8ZX | 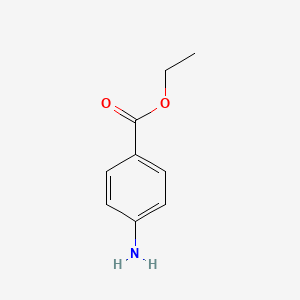 |
0.167 | ||
| ENC000391 | 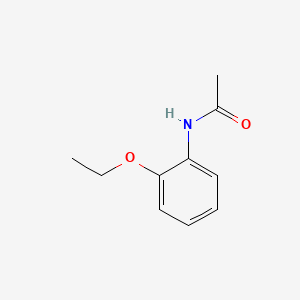 |
0.204 | D0V9JR | 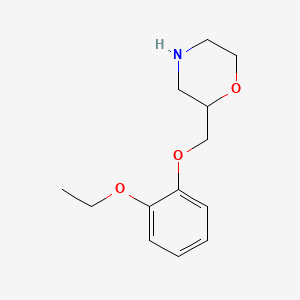 |
0.159 | ||
| ENC000657 |  |
0.200 | D01OUE | 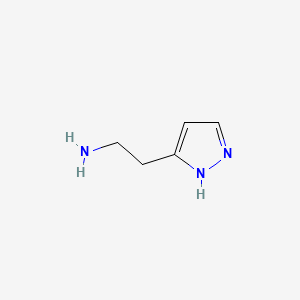 |
0.150 | ||
| ENC000785 | 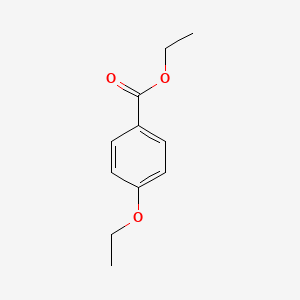 |
0.192 | D0XF8W |  |
0.143 | ||
| ENC000163 | 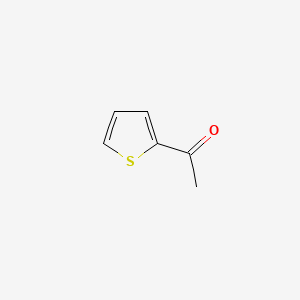 |
0.184 | D02NJA | 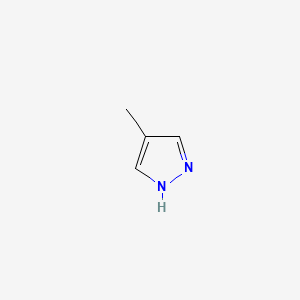 |
0.143 | ||
| ENC001061 | 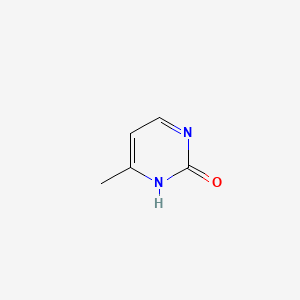 |
0.184 | D06PQT | 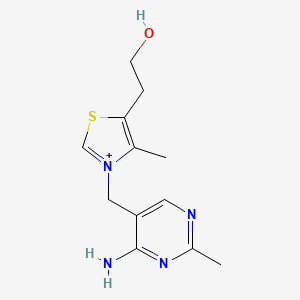 |
0.141 | ||