NPs Basic Information
|
Name |
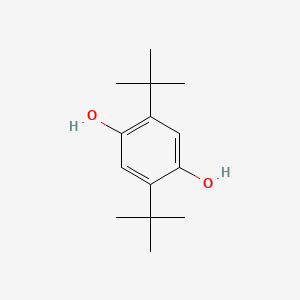
2,5-Di-tert-butylhydroquinone
|
| Molecular Formula | C14H22O2 | |
| IUPAC Name* |
2,5-ditert-butylbenzene-1,4-diol
|
|
| SMILES |
CC(C)(C)C1=CC(=C(C=C1O)C(C)(C)C)O
|
|
| InChI |
InChI=1S/C14H22O2/c1-13(2,3)9-7-12(16)10(8-11(9)15)14(4,5)6/h7-8,15-16H,1-6H3
|
|
| InChIKey |
JZODKRWQWUWGCD-UHFFFAOYSA-N
|
|
| Synonyms |
2,5-Di-tert-butylhydroquinone; 88-58-4; 2,5-Di-tert-butylbenzene-1,4-diol; DTBHQ; Dibug; Dybug; Santovar O; 2,5-Di-tert-butylquinol; Di-t-butylhydroquinone; 2,5-Di-t-butylhydroquinone; bhq; Naugard 451; 1,4-Benzenediol, 2,5-bis(1,1-dimethylethyl)-; Nonflex Alba; Antage DBH; di-tert-butylhydroquinone; Nocrac NS 7; 2,5-DITERT-BUTYLBENZENE-1,4-DIOL; 2,5-Di-tert-butyl-1,4-hydroquinone; 1,4-Dihydroxy-2,5-di-tert-butylbenzene; 2,5-Di-tert-butyl-1,4-benzenediol; 2,5-Di-tert-butyl-1,4-benzohydroquinone; NSC 11; NSC-11; HYDROQUINONE, 2,5-DI-tert-BUTYL-; 2,5-Bis(1,1-dimethylethyl)-1,4-benzenediol; 2,5-di-tert-butyl hydroquinone; CHEBI:41094; 26XK13B61B; Hydroquinone,5-di-tert-butyl-; 1, 2,5-bis(1,1-dimethylethyl)-; 2,5-di-tert-butyl-1,4-dihydroxybenzene; CCRIS 5218; WLN: L6V DVJ BX1&1&1 EX1&1&1; Hydrpquinone, 2,5-di-tert-butyl-; EINECS 201-841-8; MFCD00008825; BRN 2049542; UNII-26XK13B61B; AI3-16630; 2agv; Eastman DTBHQ; 2,5-bis(tert-butyl)benzene-1,4-diol; Tocris-1236; Cambridge id 5105618; DSSTox_CID_21248; DSSTox_RID_79662; NSC11; DSSTox_GSID_41248; Oprea1_534698; SCHEMBL38604; 2,5-di-tertbutylhydroquinone; BSPBio_001029; CBDivE_001992; KBioGR_000369; KBioSS_000369; 2,5-di-t-butyl hydroquinone; MLS001066345; 2,5-DI-(TERT-BUTYL)-1,4,BENZOHYDROQUINONE; 2,5-ditert-butyl hydroquinone; 2,5-TBHQ; CHEMBL480626; 2,5-di-tert-butyl-hydroquinone; DTXSID8041248; KBio2_000369; KBio2_002937; KBio2_005505; KBio3_000717; KBio3_000718; ZINC56404; 2,5-di-tert--butyl-hydroquinone; NSC9886; 2,5-Di-(tert-butyl)hydroquinone; BDBM176764; Bio1_000419; Bio1_000908; Bio1_001397; Bio2_000345; Bio2_000825; HMS1362C11; HMS1792C11; HMS1990C11; HMS3267F19; HMS3403C11; HMS3412A14; HMS3676A14; AMY21868; NSC-9886; Tox21_300385; CA-420; HSCI1_000289; s3628; DI-T-BUTYLHYDROQUINONE [INCI]; 2,5-di-tert--butylbenzene-1,4-diol; 2,5-Di-tert-butylhydroquinone, 99%; AKOS003627062; 2,5-Ditert-butyl-1,4-benzenediol #; AC-2488; CCG-266736; CS-W013115; DB04638; HY-W012399; PS-7822; 2,5-Di-tert-butylhydroquinone (DBHQ); CAS-88-58-4; IDI1_002100; BUTYLHYDROQUINONE, 2,5-DI-TERT-; NCGC00025066-01; NCGC00025066-02; NCGC00025066-03; NCGC00025066-04; NCGC00254338-01; CAS# 88-58-4; SMR000135117; DB-057086; D0940; FT-0610494; EN300-213340; US9688816, 7; A842719; SR-01000597417; Q-200201; SR-01000597417-1; BRD-K95603879-001-03-4; BRD-K95603879-001-06-7; Q27095374; Z1741982433
|
|
| CAS | 88-58-4 | |
| PubChem CID | 2374 | |
| ChEMBL ID | CHEMBL480626 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 222.32 | ALogp: | 4.6 |
| HBD: | 2 | HBA: | 2 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 40.5 | Aromatic Rings: | 1 |
| Heavy Atoms: | 16 | QED Weighted: | 0.639 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.103 | MDCK Permeability: | 0.00001270 |
| Pgp-inhibitor: | 0.588 | Pgp-substrate: | 0.008 |
| Human Intestinal Absorption (HIA): | 0.794 | 20% Bioavailability (F20%): | 0.998 |
| 30% Bioavailability (F30%): | 0.994 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.214 | Plasma Protein Binding (PPB): | 97.80% |
| Volume Distribution (VD): | 4.41 | Fu: | 5.18% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.911 | CYP1A2-substrate: | 0.937 |
| CYP2C19-inhibitor: | 0.504 | CYP2C19-substrate: | 0.409 |
| CYP2C9-inhibitor: | 0.542 | CYP2C9-substrate: | 0.9 |
| CYP2D6-inhibitor: | 0.899 | CYP2D6-substrate: | 0.888 |
| CYP3A4-inhibitor: | 0.357 | CYP3A4-substrate: | 0.539 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.773 | Half-life (T1/2): | 0.614 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.012 | Human Hepatotoxicity (H-HT): | 0.061 |
| Drug-inuced Liver Injury (DILI): | 0.029 | AMES Toxicity: | 0.025 |
| Rat Oral Acute Toxicity: | 0.161 | Maximum Recommended Daily Dose: | 0.888 |
| Skin Sensitization: | 0.904 | Carcinogencity: | 0.034 |
| Eye Corrosion: | 0.956 | Eye Irritation: | 0.952 |
| Respiratory Toxicity: | 0.782 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000346 | 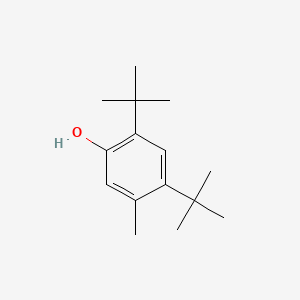 |
0.702 | D0W7WC | 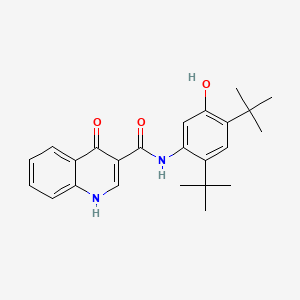 |
0.388 | ||
| ENC000725 | 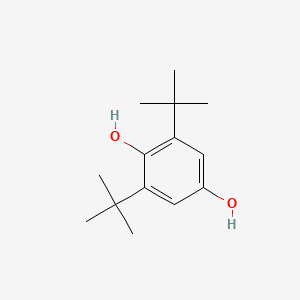 |
0.667 | D0H2DQ | 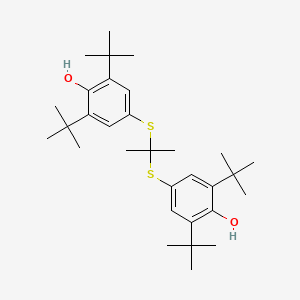 |
0.330 | ||
| ENC000610 |  |
0.600 | D00NJL | 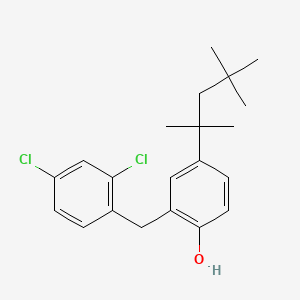 |
0.284 | ||
| ENC000708 | 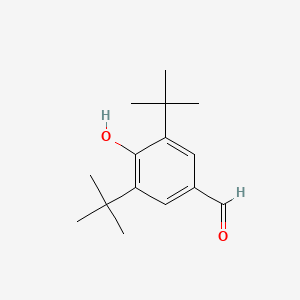 |
0.566 | D09EBS | 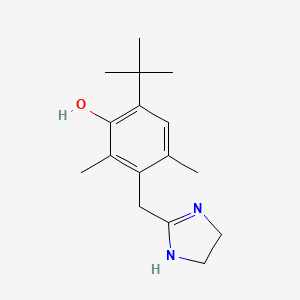 |
0.282 | ||
| ENC000185 | 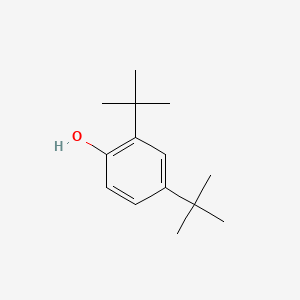 |
0.560 | D0M8RC | 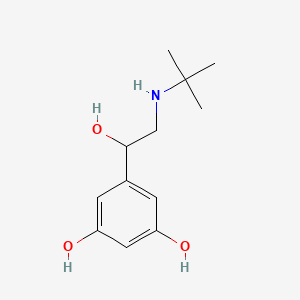 |
0.281 | ||
| ENC000744 | 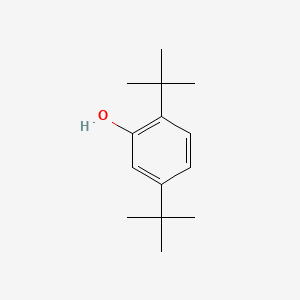 |
0.560 | D0Y4DY | 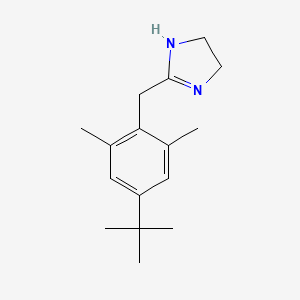 |
0.254 | ||
| ENC005113 | 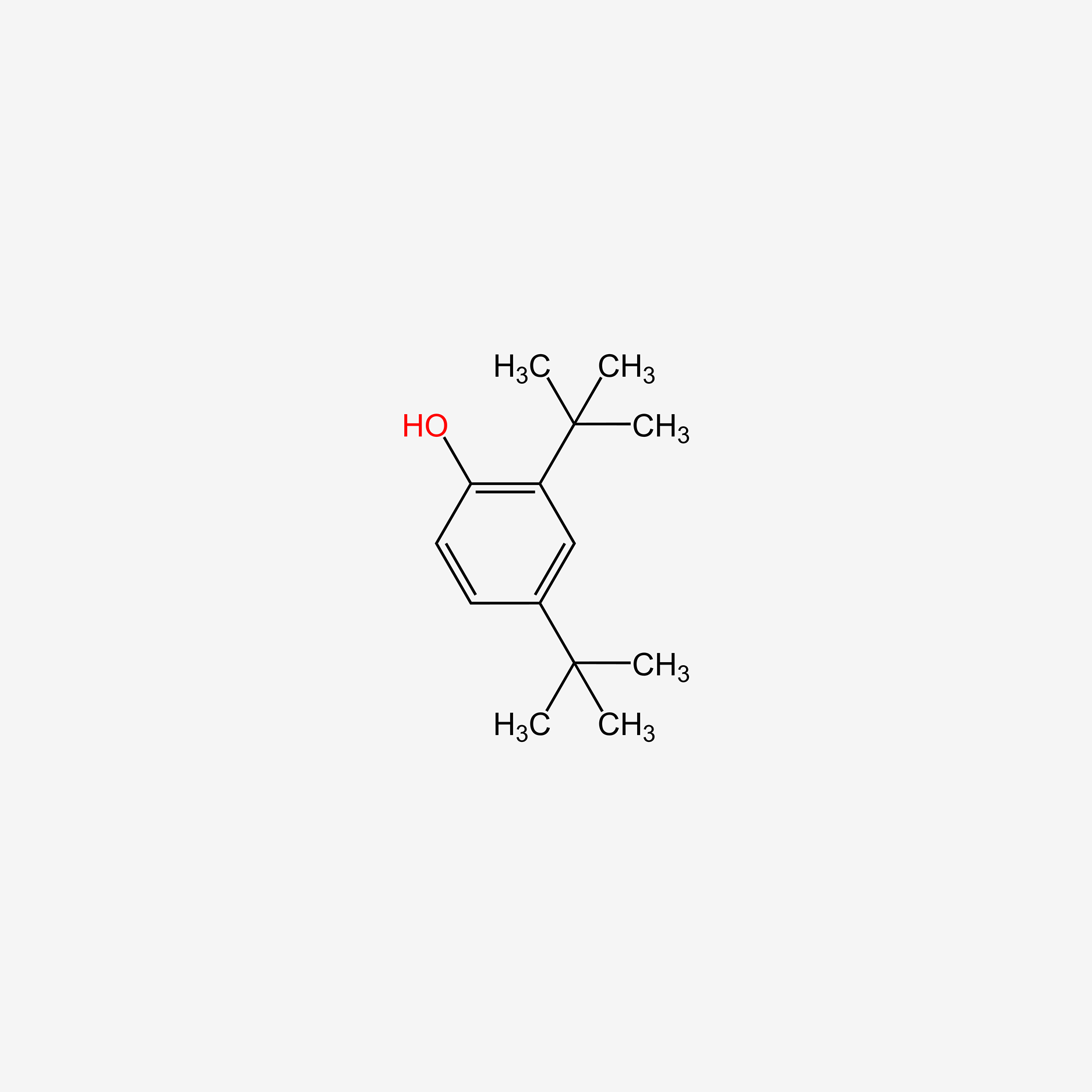 |
0.560 | D01JFT | 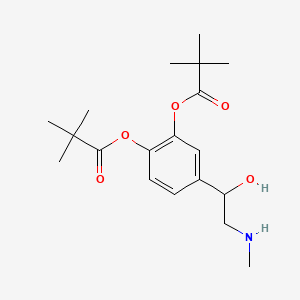 |
0.250 | ||
| ENC000611 | 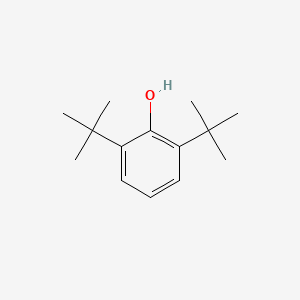 |
0.529 | D02ZJI | 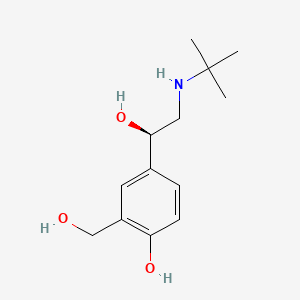 |
0.250 | ||
| ENC001398 | 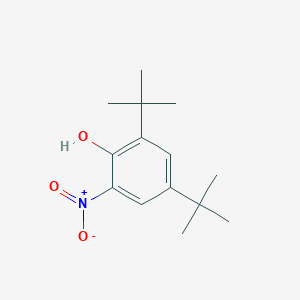 |
0.491 | D0K5CB | 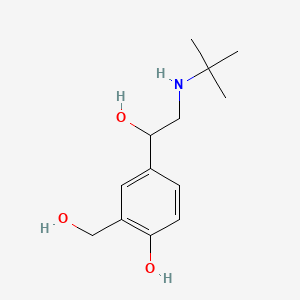 |
0.250 | ||
| ENC000695 | 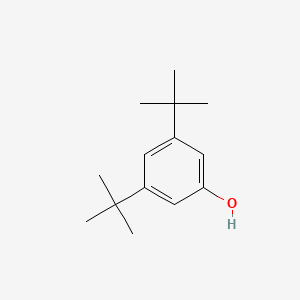 |
0.472 | D0X5NX | 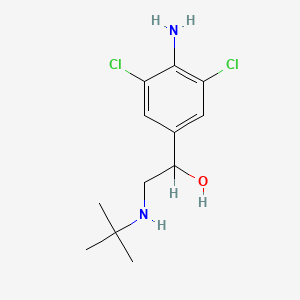 |
0.235 | ||