NPs Basic Information
|
Name |
2-Ethylhexyl acrylate
|
| Molecular Formula | C11H20O2 | |
| IUPAC Name* |
2-ethylhexyl prop-2-enoate
|
|
| SMILES |
CCCCC(CC)COC(=O)C=C
|
|
| InChI |
InChI=1S/C11H20O2/c1-4-7-8-10(5-2)9-13-11(12)6-3/h6,10H,3-5,7-9H2,1-2H3
|
|
| InChIKey |
GOXQRTZXKQZDDN-UHFFFAOYSA-N
|
|
| Synonyms |
2-ETHYLHEXYL ACRYLATE; 103-11-7; 2-ethylhexyl prop-2-enoate; 2-ETHYLHEXYLACRYLATE; 2-Propenoic acid, 2-ethylhexyl ester; 2-Ethylhexyl 2-propenoate; Acrylic acid, 2-ethylhexyl ester; 2-Ethyl-1-hexyl acrylate; 2-ethylexyl acrylate; 1-Hexanol, 2-ethyl-, acrylate; NSC 4803; 9003-77-4; Mono(2-ethylhexyl) acrylate; acrylic acid 2-ethylhexyl ester; 2-Ethylhexylester kyseliny akrylove; 2EHA; HR49R9S6XG; CHEBI:82465; NSC-4803; DSSTox_CID_5297; DSSTox_RID_77732; DSSTox_GSID_25297; 2EHA; EHA; JR 910; NSC 4803; Norsocryl 2-EHA; CAS-103-11-7; CCRIS 3430; HSDB 1121; EINECS 203-080-7; UNII-HR49R9S6XG; BRN 1765828; AI3-03833; 2-Ethylhexylester kyseliny akrylove [Czech]; ethylhexylacrylate; EINECS 215-330-2; 1-Hexanol, acrylate; Octyl Acrylate Monomer; 2-ethylhexyl propenoate; Acrylic Acid 2-Ethylhexyl Ester Monomer; 2-Ethylhexanol acrylate; JC BASE ACRYLATE; NORSOCRYL 2-EHA; Acrylic acid 2-ethylhexyl; EC 203-080-7; 2-Ethylhexyl Acrylate Resin; SCHEMBL14869; 2-Ethylhexyl Acrylate Monomer; Acrylic acid-2-ethylhexyl ester; CHEMBL1574328; DTXSID9025297; Acrylic Acid Octyl Ester Monomer; NSC4803; ETHYLHEXYL ACRYLATE [INCI]; 2-Ethylhexyl ester of acrylic acid; Tox21_202053; Tox21_303227; WLN: 4Y2 & 1OV1U1; MFCD00084372; 2-ETHYLHEXYL ACRYLATE [IARC]; AKOS015894409; (+/-)-Acrylic acid 2-ethylhexyl ester; NCGC00091115-01; NCGC00091115-02; NCGC00091115-03; NCGC00256960-01; NCGC00259602-01; LS-14013; 2-Ethylhexyl acrylate, analytical standard; DB-030721; A0144; FT-0612226; 2-Ethylhexyl Acrylate Monomer, stab. w/MEHQ; C19420; A896619; Q209383; Q-200277; 2-Ethylhexyl Acrylate Monomer (stabilized with MEHQ); 2-Ethylhexyl acrylate, 98%, contains >=0.001-<=0.11% monomethyl ether hydroquinone as stabilizer
|
|
| CAS | 103-11-7 | |
| PubChem CID | 7636 | |
| ChEMBL ID | CHEMBL1574328 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 184.27 | ALogp: | 3.8 |
| HBD: | 0 | HBA: | 2 |
| Rotatable Bonds: | 8 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 26.3 | Aromatic Rings: | 0 |
| Heavy Atoms: | 13 | QED Weighted: | 0.445 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.331 | MDCK Permeability: | 0.00002960 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.007 |
| Human Intestinal Absorption (HIA): | 0.002 | 20% Bioavailability (F20%): | 0.027 |
| 30% Bioavailability (F30%): | 0.045 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.984 | Plasma Protein Binding (PPB): | 83.94% |
| Volume Distribution (VD): | 0.84 | Fu: | 11.80% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.871 | CYP1A2-substrate: | 0.652 |
| CYP2C19-inhibitor: | 0.469 | CYP2C19-substrate: | 0.757 |
| CYP2C9-inhibitor: | 0.501 | CYP2C9-substrate: | 0.463 |
| CYP2D6-inhibitor: | 0.205 | CYP2D6-substrate: | 0.271 |
| CYP3A4-inhibitor: | 0.256 | CYP3A4-substrate: | 0.222 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.657 | Half-life (T1/2): | 0.65 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.008 | Human Hepatotoxicity (H-HT): | 0.049 |
| Drug-inuced Liver Injury (DILI): | 0.033 | AMES Toxicity: | 0.01 |
| Rat Oral Acute Toxicity: | 0.036 | Maximum Recommended Daily Dose: | 0.141 |
| Skin Sensitization: | 0.949 | Carcinogencity: | 0.399 |
| Eye Corrosion: | 0.99 | Eye Irritation: | 0.979 |
| Respiratory Toxicity: | 0.794 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
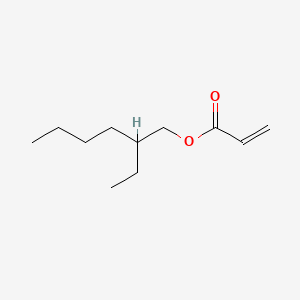
| ENC000211 | 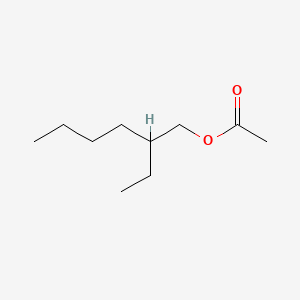 |
0.675 | D0X4FM | 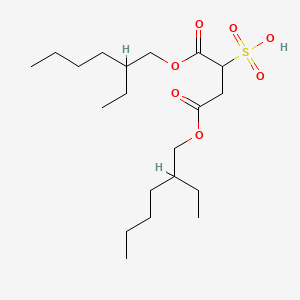 |
0.325 | ||
| ENC000652 | 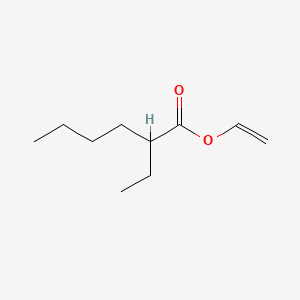 |
0.489 | D0Y3KG | 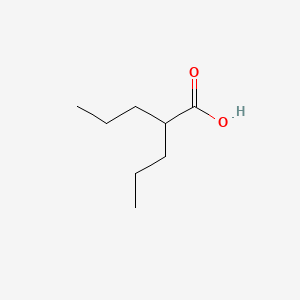 |
0.298 | ||
| ENC001899 | 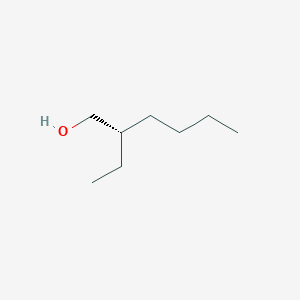 |
0.475 | D0AY9Q | 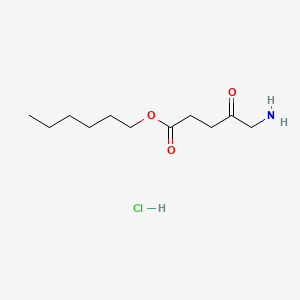 |
0.242 | ||
| ENC000220 | 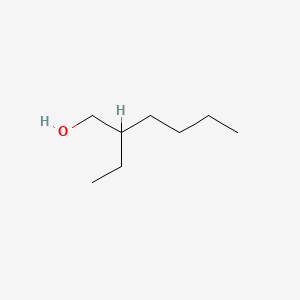 |
0.475 | D01QLH | 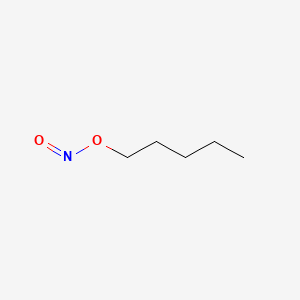 |
0.239 | ||
| ENC000543 | 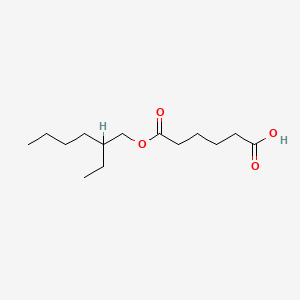 |
0.474 | D08HQK | 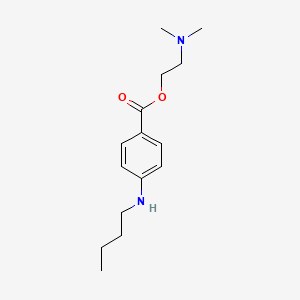 |
0.222 | ||
| ENC000544 | 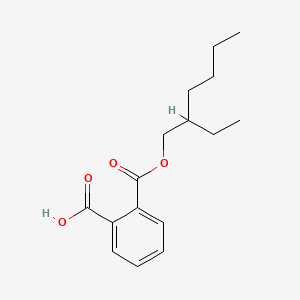 |
0.429 | D0ZK8H | 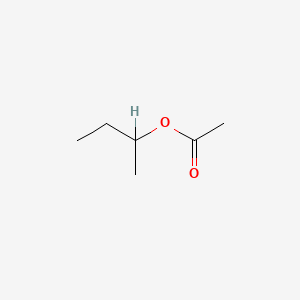 |
0.222 | ||
| ENC000833 | 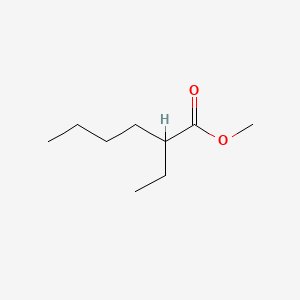 |
0.422 | D0H2SY | 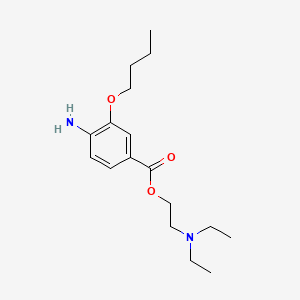 |
0.215 | ||
| ENC000306 | 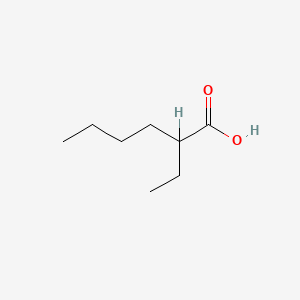 |
0.419 | D0Y4AW | 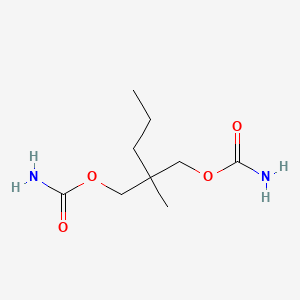 |
0.213 | ||
| ENC001211 | 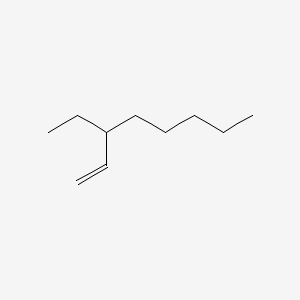 |
0.409 | D06ORU | 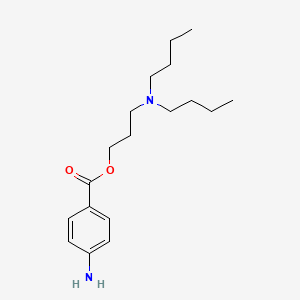 |
0.213 | ||
| ENC003073 | 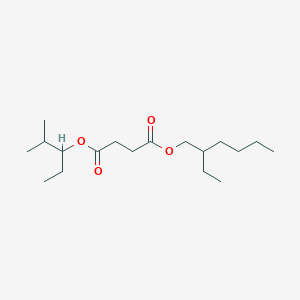 |
0.403 | D0CT4D | 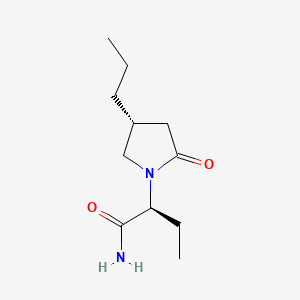 |
0.210 | ||