NPs Basic Information
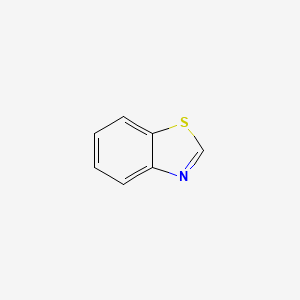
|
Name |
Benzothiazole
|
| Molecular Formula | C7H5NS | |
| IUPAC Name* |
1,3-benzothiazole
|
|
| SMILES |
C1=CC=C2C(=C1)N=CS2
|
|
| InChI |
InChI=1S/C7H5NS/c1-2-4-7-6(3-1)8-5-9-7/h1-5H
|
|
| InChIKey |
IOJUPLGTWVMSFF-UHFFFAOYSA-N
|
|
| Synonyms |
BENZOTHIAZOLE; 95-16-9; BENZO[D]THIAZOLE; 1,3-Benzothiazole; Benzosulfonazole; 1-Thia-3-azaindene; Vangard BT; benzothiazol; USAF EK-4812; FEMA No. 3256; CHEBI:45993; O-2857; G5BW2593EP; NSC-8040; BT; DSSTox_CID_4586; DSSTox_RID_77458; DSSTox_GSID_24586; benzthiazole; FEMA Number 3256; CAS-95-16-9; CCRIS 7893; HSDB 2796; NSC 8040; EINECS 202-396-2; MFCD00005775; BRN 0109468; UNII-G5BW2593EP; s-benzothiazole; AI3-05742; Benzothiazole, 96%; 1,3-Benzothiazole #; BENZOTHIAZOLE [MI]; Epitope ID:138946; EC 202-396-2; SCHEMBL8430; BENZOTHIAZOLE [FHFI]; BENZOTHIAZOLE [HSDB]; WLN: T56 BN DSJ; 4-27-00-01069 (Beilstein Handbook Reference); MLS001050134; Benzothiazole, >=96%, FG; CHEMBL510309; SCHEMBL9304593; DTXSID7024586; ZINC19726; NSC8040; Benzothiazole, analytical standard; AMY23315; Tox21_201853; Tox21_303232; BDBM50444460; LT0034; STL268890; AKOS000120178; AC-3297; CS-W013350; FS-4155; HY-W012634; NCGC00091399-01; NCGC00091399-02; NCGC00257070-01; NCGC00259402-01; BOT; SMR001216577; DB-057562; B0092; FT-0622731; FT-0662581; Benzothiazole, Vetec(TM) reagent grade, 96%; EN300-19148; D77749; AC-907/25014160; Q419096; Q-100900; F0001-2268; Z104472964
|
|
| CAS | 95-16-9 | |
| PubChem CID | 7222 | |
| ChEMBL ID | CHEMBL510309 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 135.19 | ALogp: | 2.0 |
| HBD: | 0 | HBA: | 2 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 41.1 | Aromatic Rings: | 2 |
| Heavy Atoms: | 9 | QED Weighted: | 0.541 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.278 | MDCK Permeability: | 0.00003070 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.866 |
| 30% Bioavailability (F30%): | 0.701 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.535 | Plasma Protein Binding (PPB): | 80.46% |
| Volume Distribution (VD): | 2.43 | Fu: | 17.36% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.991 | CYP1A2-substrate: | 0.852 |
| CYP2C19-inhibitor: | 0.507 | CYP2C19-substrate: | 0.524 |
| CYP2C9-inhibitor: | 0.09 | CYP2C9-substrate: | 0.5 |
| CYP2D6-inhibitor: | 0.856 | CYP2D6-substrate: | 0.668 |
| CYP3A4-inhibitor: | 0.053 | CYP3A4-substrate: | 0.36 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 9.523 | Half-life (T1/2): | 0.593 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.023 | Human Hepatotoxicity (H-HT): | 0.074 |
| Drug-inuced Liver Injury (DILI): | 0.87 | AMES Toxicity: | 0.435 |
| Rat Oral Acute Toxicity: | 0.145 | Maximum Recommended Daily Dose: | 0.029 |
| Skin Sensitization: | 0.353 | Carcinogencity: | 0.293 |
| Eye Corrosion: | 0.711 | Eye Irritation: | 0.991 |
| Respiratory Toxicity: | 0.919 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000784 | 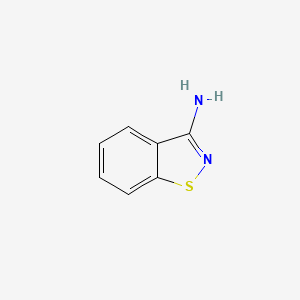 |
0.400 | D08QCJ | 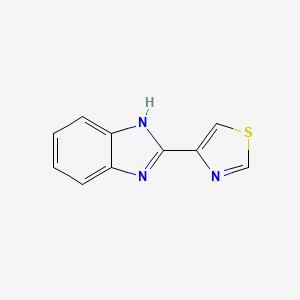 |
0.380 | ||
| ENC000892 | 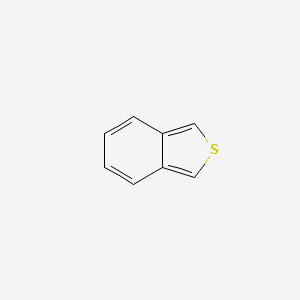 |
0.350 | D05OIS |  |
0.316 | ||
| ENC000064 | 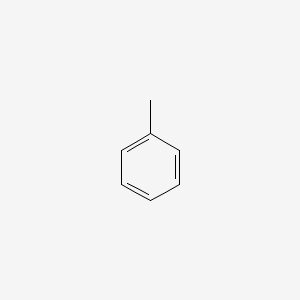 |
0.343 | D0X9RY |  |
0.300 | ||
| ENC000052 | 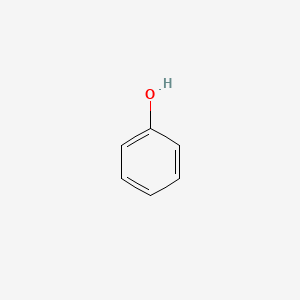 |
0.343 | D0K1XK | 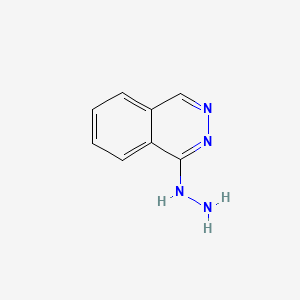 |
0.292 | ||
| ENC000179 | 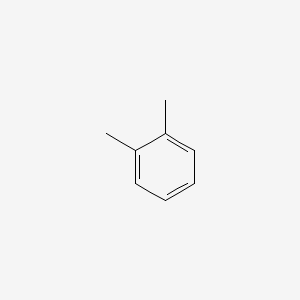 |
0.324 | D09JUG | 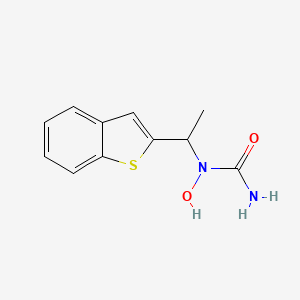 |
0.291 | ||
| ENC000021 | 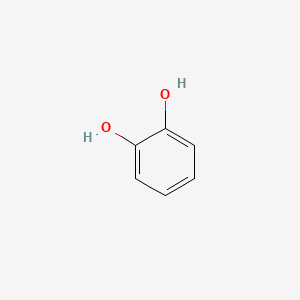 |
0.324 | D03RZV | 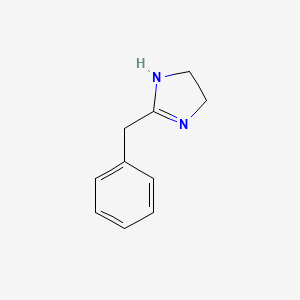 |
0.286 | ||
| ENC000028 | 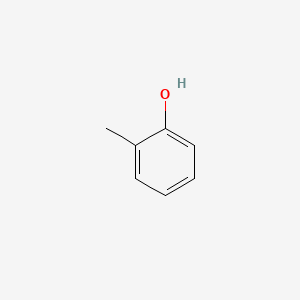 |
0.324 | D07HBX |  |
0.286 | ||
| ENC000041 |  |
0.317 | D0D5GG | 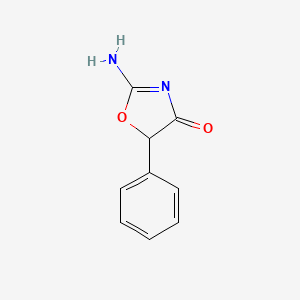 |
0.280 | ||
| ENC000204 | 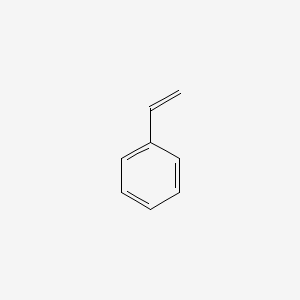 |
0.316 | D06DLI | 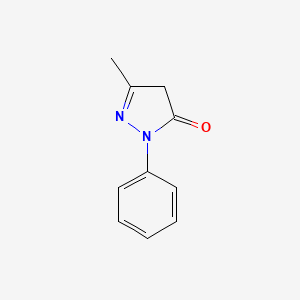 |
0.280 | ||
| ENC000012 | 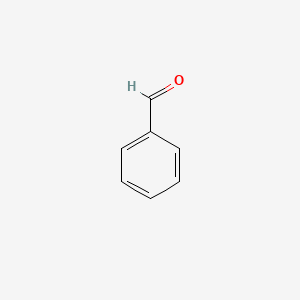 |
0.316 | D0H0HJ | 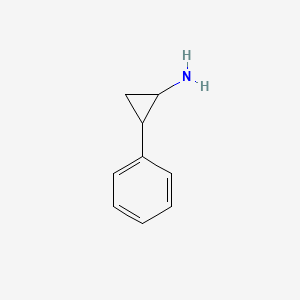 |
0.279 | ||