NPs Basic Information

|
Name |
Indole
|
| Molecular Formula | C8H7N | |
| IUPAC Name* |
1H-indole
|
|
| SMILES |
C1=CC=C2C(=C1)C=CN2
|
|
| InChI |
InChI=1S/C8H7N/c1-2-4-8-7(3-1)5-6-9-8/h1-6,9H
|
|
| InChIKey |
SIKJAQJRHWYJAI-UHFFFAOYSA-N
|
|
| Synonyms |
indole; 1H-Indole; 120-72-9; 2,3-Benzopyrrole; Indol; 1-Benzazole; Ketole; 1-Azaindene; Benzopyrrole; 2,3-Benzopyrole; Caswell No. 498B; Indol [German]; Indole (natural); 1-Benzo(b)pyrrole; 1H-Benzo[b]pyrrole; FEMA No. 2593; CCRIS 4421; HSDB 599; EPA Pesticide Chemical Code 025000; Benzo[b]pyrrole; AI3-01540; MFCD00005607; CHEMBL15844; CHEBI:16881; 8724FJW4M5; NSC-1964; Indole 100 microg/mL in Acetonitrile; NCGC00167539-01; DSSTox_CID_737; DSSTox_RID_75761; DSSTox_GSID_20737; 82451-55-6; IND; CAS-120-72-9; NSC 1964; EINECS 204-420-7; benzazole; mono-indole; UNII-8724FJW4M5; 1-H-indole; Indole, 7; INDOLUM; Indole (8CI); Indole (white flake); Indole, 98%; 1H-Indole (9CI); INDOLUM [HPUS]; INDOLE [FHFI]; INDOLE [HSDB]; INDOLE [FCC]; INDOLE [USP-RS]; INDOLE [MI]; Indole, >=99%; SCHEMBL698; bmse000097; Indole, analytical standard; Indole, >=99%, FG; WLN: T56 BMJ; BIDD:GT0304; SCHEMBL940818; INDOLE BENZO-PYRROLE; SCHEMBL1921769; SCHEMBL9559244; DTXSID0020737; AMY3411; NSC1964; 185l; BCP27232; STR01201; Tox21_112536; Tox21_201677; Tox21_302937; BBL011739; BDBM50094702; s6358; STL163380; ZINC14516984; AKOS000119629; Tox21_112536_1; CG-0501; CS-W001132; DB04532; HY-W001132; Indole, puriss., >=98.5% (GC); NCGC00167539-02; NCGC00167539-03; NCGC00256348-01; NCGC00259226-01; BP-10563; DS-011308; FT-0627211; I0021; EN300-18285; C00463; I-0800; I-0810; Q319541; SR-01000944736; SR-01000944736-1; Z57833933; F2190-0647
|
|
| CAS | 120-72-9 | |
| PubChem CID | 798 | |
| ChEMBL ID | CHEMBL15844 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 117.15 | ALogp: | 2.1 |
| HBD: | 1 | HBA: | 0 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 15.8 | Aromatic Rings: | 2 |
| Heavy Atoms: | 9 | QED Weighted: | 0.546 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.259 | MDCK Permeability: | 0.00002250 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.012 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.881 |
| 30% Bioavailability (F30%): | 0.468 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.737 | Plasma Protein Binding (PPB): | 86.32% |
| Volume Distribution (VD): | 1.902 | Fu: | 16.50% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.975 | CYP1A2-substrate: | 0.805 |
| CYP2C19-inhibitor: | 0.789 | CYP2C19-substrate: | 0.302 |
| CYP2C9-inhibitor: | 0.146 | CYP2C9-substrate: | 0.811 |
| CYP2D6-inhibitor: | 0.335 | CYP2D6-substrate: | 0.854 |
| CYP3A4-inhibitor: | 0.078 | CYP3A4-substrate: | 0.207 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 11.189 | Half-life (T1/2): | 0.794 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.029 | Human Hepatotoxicity (H-HT): | 0.178 |
| Drug-inuced Liver Injury (DILI): | 0.406 | AMES Toxicity: | 0.311 |
| Rat Oral Acute Toxicity: | 0.844 | Maximum Recommended Daily Dose: | 0.219 |
| Skin Sensitization: | 0.633 | Carcinogencity: | 0.408 |
| Eye Corrosion: | 0.844 | Eye Irritation: | 0.994 |
| Respiratory Toxicity: | 0.983 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000178 | 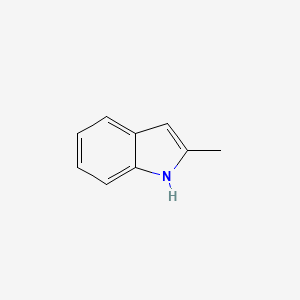 |
0.474 | D08QCJ | 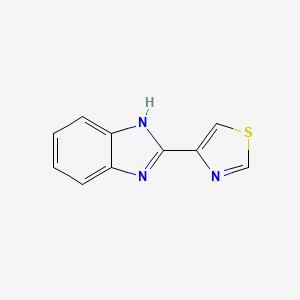 |
0.327 | ||
| ENC000047 | 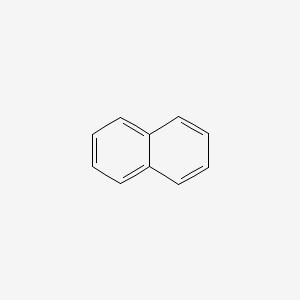 |
0.425 | D05EJG | 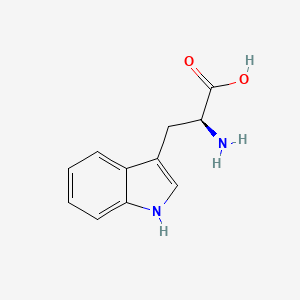 |
0.327 | ||
| ENC000892 | 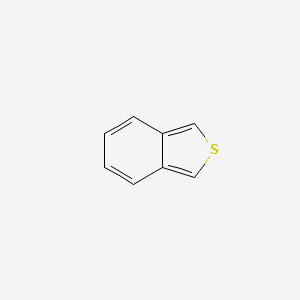 |
0.421 | D0K1XK | 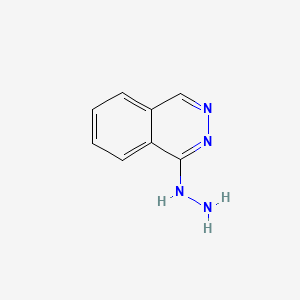 |
0.319 | ||
| ENC000675 | 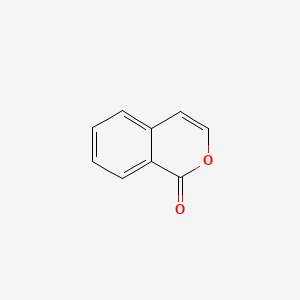 |
0.405 | D0O6IZ | 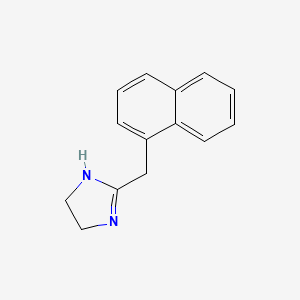 |
0.316 | ||
| ENC000025 | 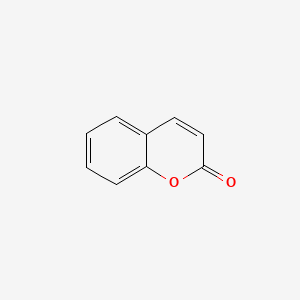 |
0.405 | D05OIS |  |
0.316 | ||
| ENC000167 | 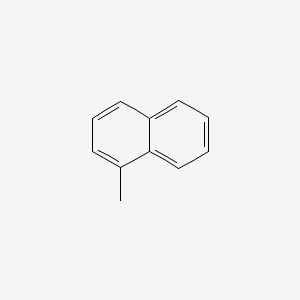 |
0.405 | D0F5ZM | 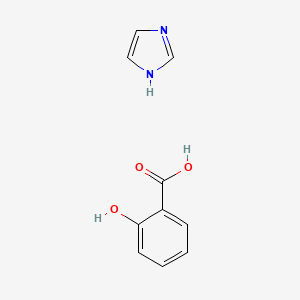 |
0.302 | ||
| ENC000169 | 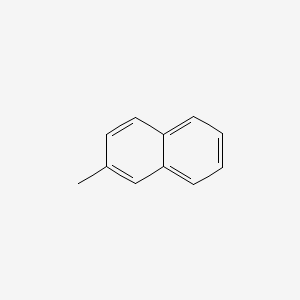 |
0.405 | D0X9RY |  |
0.300 | ||
| ENC000341 |  |
0.405 | D0F2PO | 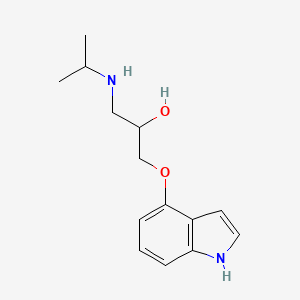 |
0.300 | ||
| ENC005757 | 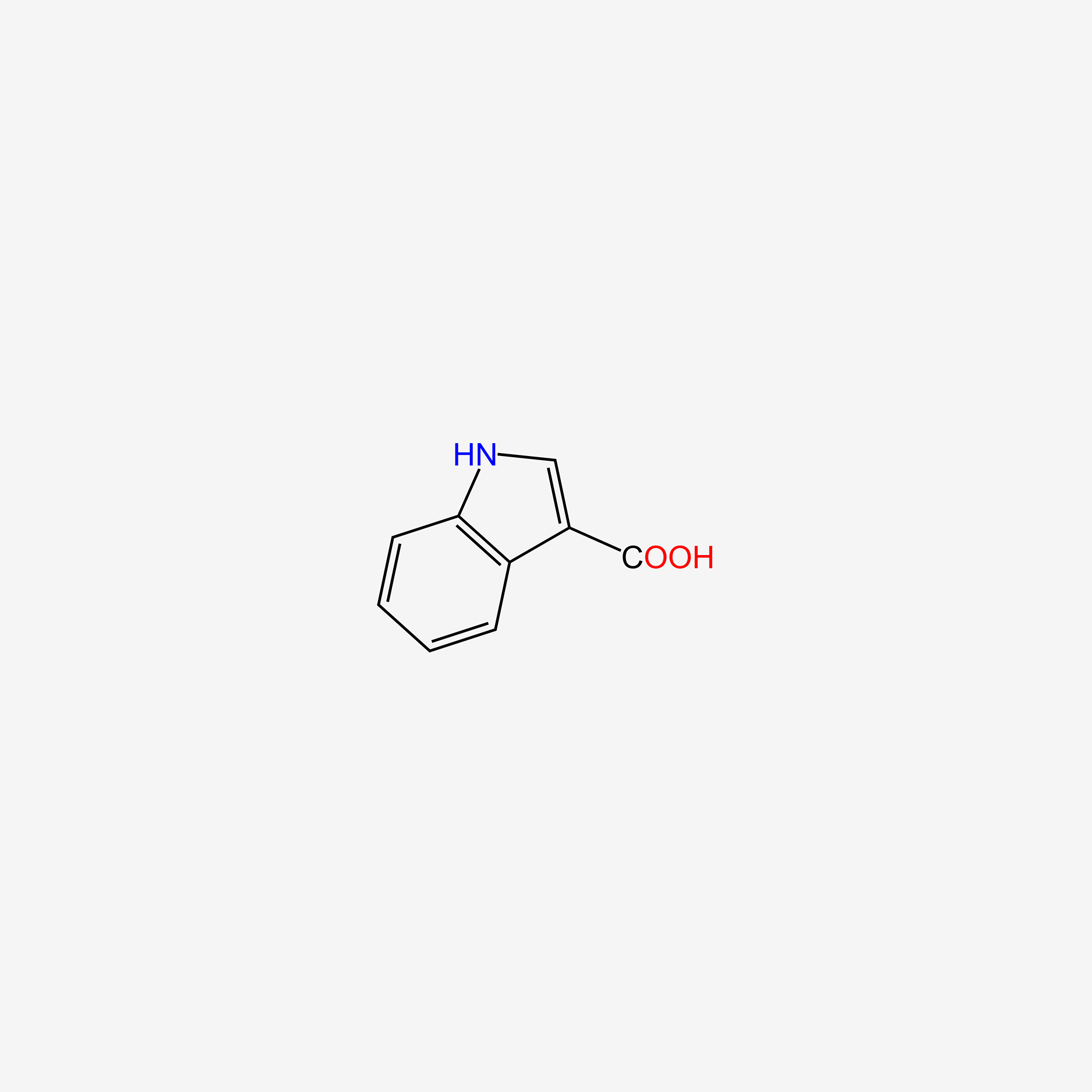 |
0.386 | D02WCI | 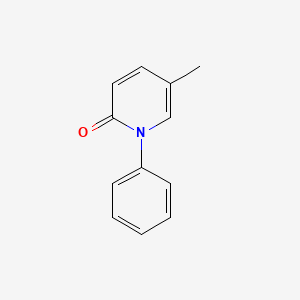 |
0.288 | ||
| ENC000714 | 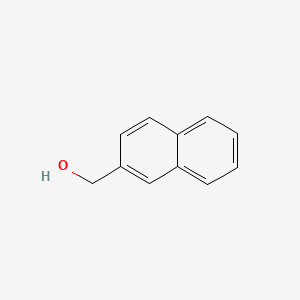 |
0.378 | D03RZV | 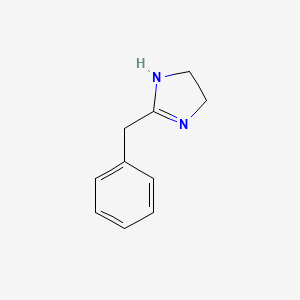 |
0.286 | ||