NPs Basic Information
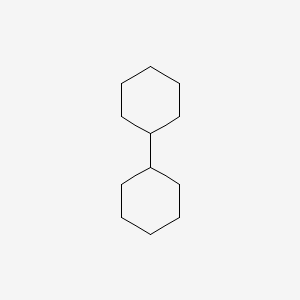
|
Name |
Bicyclohexyl
|
| Molecular Formula | C12H22 | |
| IUPAC Name* |
cyclohexylcyclohexane
|
|
| SMILES |
C1CCC(CC1)C2CCCCC2
|
|
| InChI |
InChI=1S/C12H22/c1-3-7-11(8-4-1)12-9-5-2-6-10-12/h11-12H,1-10H2
|
|
| InChIKey |
WVIIMZNLDWSIRH-UHFFFAOYSA-N
|
|
| Synonyms |
Bicyclohexyl; 92-51-3; 1,1'-Bicyclohexyl; DICYCLOHEXYL; Cyclohexylcyclohexane; Bicyclohexane; Dicyclohexane; Dodecahydrobiphenyl; Cyclohexane, cyclohexyl-; 1,1'-Biphenyl, dodecahydro-; NSC 59855; MFCD00003815; NSC-59855; Y77501141O; 1,1'-BI(CYCLOHEXYL); 1,1'-Bi(cyclohexane); EINECS 202-161-4; bi(cyclohexane); AI3-01174; 1, dodecahydro-; cyclohexylcyclohexan; UNII-Y77501141O; 1,1-Bicyclohexyl; Bicyclohexyl, 99%; Cyclohexyl Cyclohexane; DSSTox_CID_1802; DSSTox_RID_76336; DSSTox_GSID_21802; CHEMBL1231413; DTXSID8021802; NSC59855; ZINC1689826; Tox21_302104; AKOS005207082; ZINC100508964; CAS-92-51-3; NCGC00255206-01; BS-29633; SY012941; DB-057310; B0902; CS-0081506; FT-0624222; N10369; W-109341; Q21099094; Dicyclohexyl, United States Pharmacopeia (USP) Reference Standard
|
|
| CAS | 92-51-3 | |
| PubChem CID | 7094 | |
| ChEMBL ID | CHEMBL1231413 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 166.3 | ALogp: | 5.7 |
| HBD: | 0 | HBA: | 0 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 0.0 | Aromatic Rings: | 2 |
| Heavy Atoms: | 12 | QED Weighted: | 0.528 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.602 | MDCK Permeability: | 0.00001580 |
| Pgp-inhibitor: | 0.004 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.002 | 20% Bioavailability (F20%): | 0.038 |
| 30% Bioavailability (F30%): | 0.754 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.217 | Plasma Protein Binding (PPB): | 97.50% |
| Volume Distribution (VD): | 2.915 | Fu: | 1.11% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.376 | CYP1A2-substrate: | 0.408 |
| CYP2C19-inhibitor: | 0.482 | CYP2C19-substrate: | 0.167 |
| CYP2C9-inhibitor: | 0.304 | CYP2C9-substrate: | 0.816 |
| CYP2D6-inhibitor: | 0.39 | CYP2D6-substrate: | 0.115 |
| CYP3A4-inhibitor: | 0.293 | CYP3A4-substrate: | 0.148 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 5.179 | Half-life (T1/2): | 0.07 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.109 | Human Hepatotoxicity (H-HT): | 0.078 |
| Drug-inuced Liver Injury (DILI): | 0.788 | AMES Toxicity: | 0.02 |
| Rat Oral Acute Toxicity: | 0.061 | Maximum Recommended Daily Dose: | 0.028 |
| Skin Sensitization: | 0.935 | Carcinogencity: | 0.058 |
| Eye Corrosion: | 0.992 | Eye Irritation: | 0.99 |
| Respiratory Toxicity: | 0.309 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001169 |  |
0.658 | D00SBN |  |
0.412 | ||
| ENC000840 |  |
0.383 | D04URO | 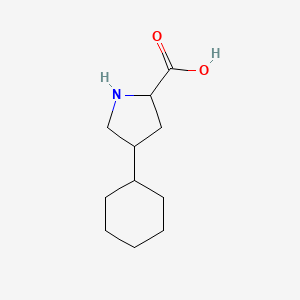 |
0.407 | ||
| ENC000323 | 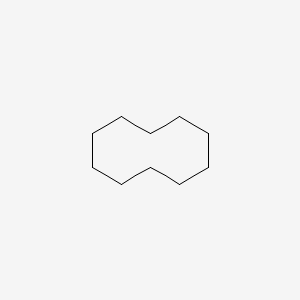 |
0.375 | D08VSI | 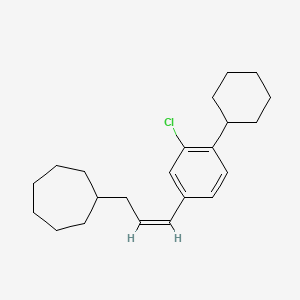 |
0.368 | ||
| ENC000251 | 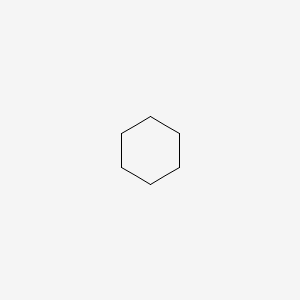 |
0.350 | D0L0MK |  |
0.338 | ||
| ENC000324 |  |
0.333 | D07XJM |  |
0.303 | ||
| ENC004910 |  |
0.328 | D09GFL | 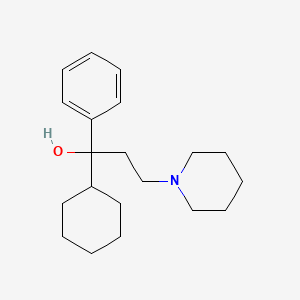 |
0.295 | ||
| ENC000893 | 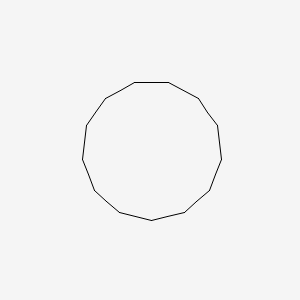 |
0.316 | D0S5NG | 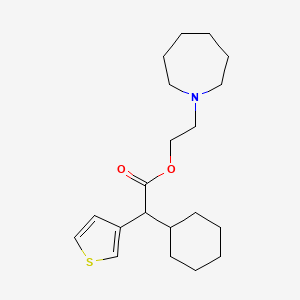 |
0.274 | ||
| ENC005710 | 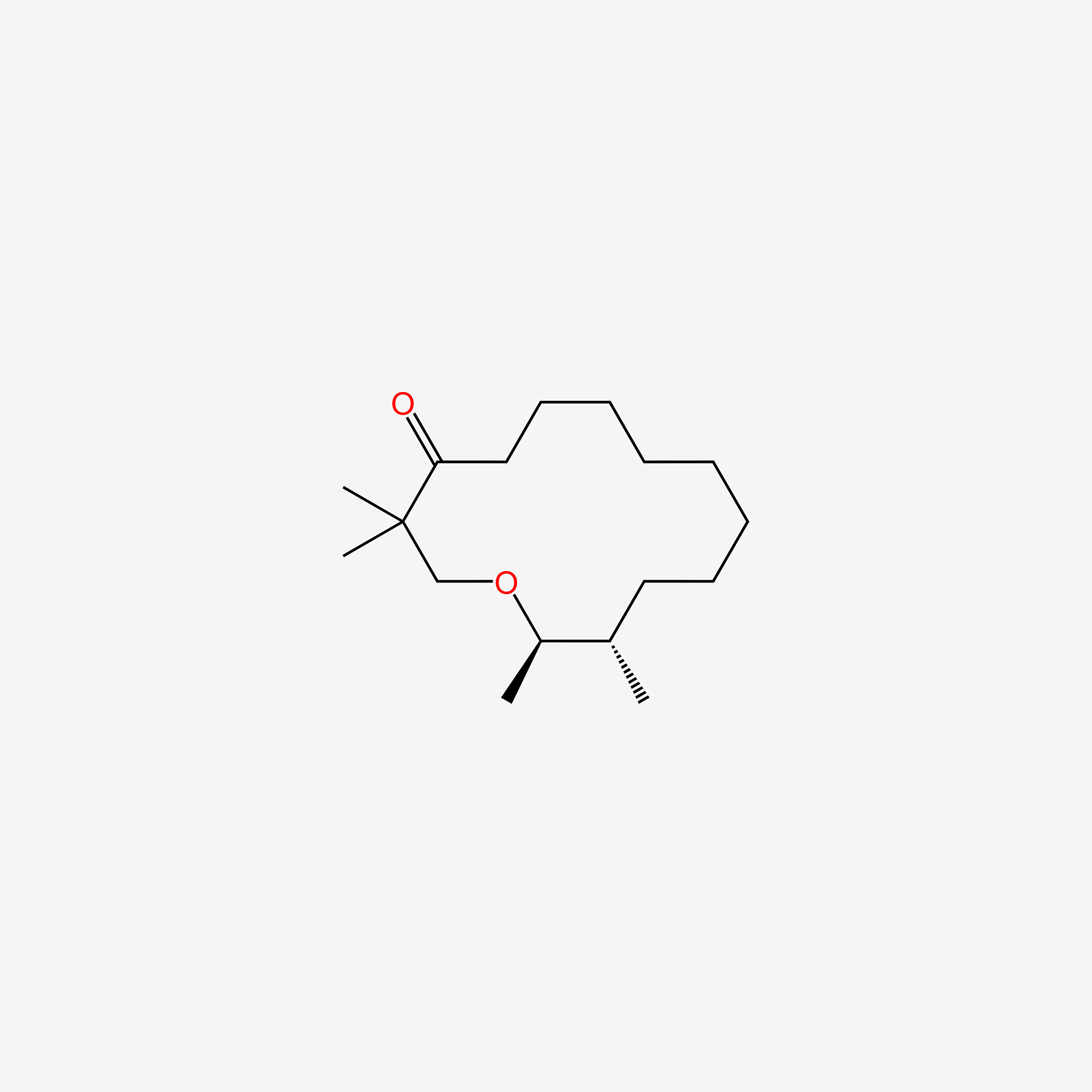 |
0.313 | D03DVJ |  |
0.264 | ||
| ENC004377 |  |
0.284 | D0N4PZ |  |
0.258 | ||
| ENC003404 |  |
0.284 | D0R1WR | 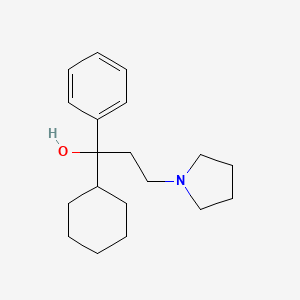 |
0.256 | ||