NPs Basic Information
|
Name |
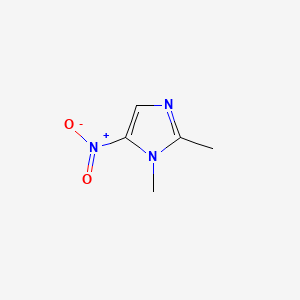
Dimetridazole
|
| Molecular Formula | C5H7N3O2 | |
| IUPAC Name* |
1,2-dimethyl-5-nitroimidazole
|
|
| SMILES |
CC1=NC=C(N1C)[N+](=O)[O-]
|
|
| InChI |
InChI=1S/C5H7N3O2/c1-4-6-3-5(7(4)2)8(9)10/h3H,1-2H3
|
|
| InChIKey |
IBXPYPUJPLLOIN-UHFFFAOYSA-N
|
|
| Synonyms |
dimetridazole; 1,2-Dimethyl-5-nitroimidazole; 551-92-8; 1,2-DIMETHYL-5-NITRO-1H-IMIDAZOLE; Dimetridazol; Emtryl; Emtrylvet; Emtrymix; Unizole Soluble; Dimetridazolum; 5-Nitro-1,2-dimethylimidazole; 1H-Imidazole, 1,2-dimethyl-5-nitro-; RP 8595; Dimetridazole free base; Alazol; NSC 226253; 1,2-dimethyl-5-nitro-imidazole; 8595 R.P.; Imidazole, 1,2-dimethyl-5-nitro-; NSC-226253; K59P7XNB8X; 551-92-8 (free base); NSC226253; Dimetridazole (INN); RP-8595; Dimetridazolo; DSSTox_CID_497; DIMETRIDAZOLE [INN]; DSSTox_RID_75623; DSSTox_GSID_20497; Dimetridazolo [DCIT]; Caswell No. 371A; Dimetridazole [INN:BAN]; Dimetridazol [INN-Spanish]; Dimetridazolum [INN-Latin]; CAS-551-92-8; CCRIS 997; EINECS 209-001-2; UNII-K59P7XNB8X; EPA Pesticide Chemical Code 371200; BRN 0130665; Demetridazole; Dimetrizadole; AI3-27217; component of Emtryl; MFCD00047046; DIMETRIDAZOLE [MI]; Alazol [veterinary] (TN); SCHEMBL63278; 5-23-05-00058 (Beilstein Handbook Reference); CHEMBL38938; DIMETRIDAZOLE [MART.]; Imidazole,2-dimethyl-5-nitro-; ZINC1307; DTXSID5020497; 1, 2-Dimethyl-5-nitroimidazole; CHEBI:141155; 1H-Imidazole,2-dimethyl-5-nitro-; ACT06679; HY-B1244; WLN: T5N CNJ A1 B1 ENW; Tox21_113657; Tox21_201618; Tox21_302989; BBL028012; s5541; STL259130; AKOS000121461; AM87060; CCG-266155; CS-4956; NCGC00249084-01; NCGC00249084-02; NCGC00256615-01; NCGC00259167-01; (1R,3S)-(-)-CAMPHORICANHYDRIDE; AC-12771; D1564; D4081; EU-0033396; FT-0606425; FT-0667567; Dimetridazole 1000 microg/mL in Acetonitrile; EN300-21183; D07855; D78134; A830512; Dimetridazole, VETRANAL(TM), analytical standard; SR-01000944778; Q5277356; SR-01000944778-1; W-105582; W-205401; Z104493616; 1,2-dimethyl-5-nitroimidazole; dimetridazole; 1,2-dimethyl-5-nitro-1h-imidazole
|
|
| CAS | 551-92-8 | |
| PubChem CID | 3090 | |
| ChEMBL ID | CHEMBL38938 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 141.13 | ALogp: | 0.1 |
| HBD: | 0 | HBA: | 3 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 63.6 | Aromatic Rings: | 1 |
| Heavy Atoms: | 10 | QED Weighted: | 0.433 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.059 | MDCK Permeability: | 0.00019199 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.014 |
| Human Intestinal Absorption (HIA): | 0.006 | 20% Bioavailability (F20%): | 0.002 |
| 30% Bioavailability (F30%): | 0 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.892 | Plasma Protein Binding (PPB): | 26.70% |
| Volume Distribution (VD): | 1.206 | Fu: | 64.21% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.08 | CYP1A2-substrate: | 0.91 |
| CYP2C19-inhibitor: | 0.036 | CYP2C19-substrate: | 0.744 |
| CYP2C9-inhibitor: | 0.007 | CYP2C9-substrate: | 0.693 |
| CYP2D6-inhibitor: | 0.004 | CYP2D6-substrate: | 0.614 |
| CYP3A4-inhibitor: | 0.006 | CYP3A4-substrate: | 0.539 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.184 | Half-life (T1/2): | 0.521 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.024 | Human Hepatotoxicity (H-HT): | 0.917 |
| Drug-inuced Liver Injury (DILI): | 0.438 | AMES Toxicity: | 0.971 |
| Rat Oral Acute Toxicity: | 0.267 | Maximum Recommended Daily Dose: | 0.043 |
| Skin Sensitization: | 0.803 | Carcinogencity: | 0.976 |
| Eye Corrosion: | 0.05 | Eye Irritation: | 0.278 |
| Respiratory Toxicity: | 0.167 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000871 | 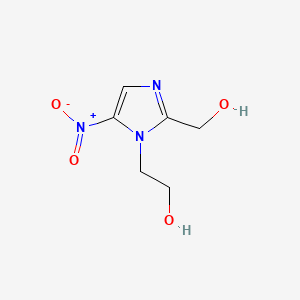 |
0.356 | D0V5IW | 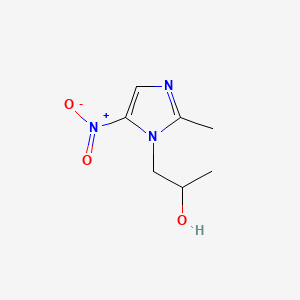 |
0.538 | ||
| ENC000599 | 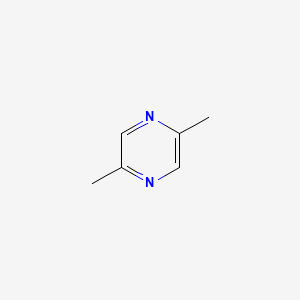 |
0.231 | D0A2ZX | 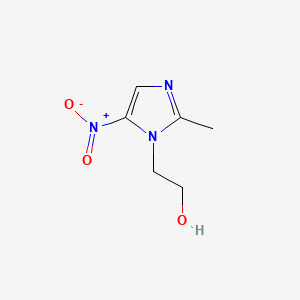 |
0.526 | ||
| ENC001348 | 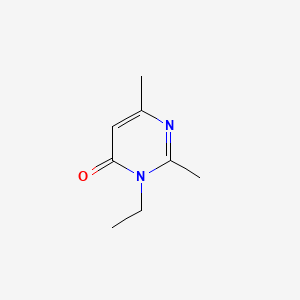 |
0.222 | D0O4SE |  |
0.447 | ||
| ENC000657 |  |
0.216 | D0S1NZ | 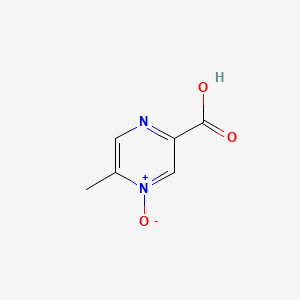 |
0.222 | ||
| ENC001061 | 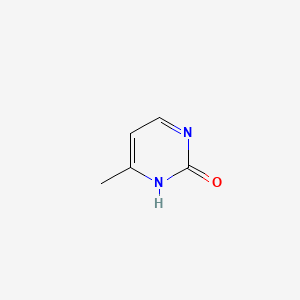 |
0.200 | D0F8RA | 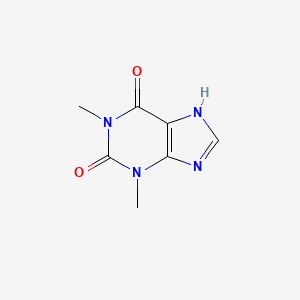 |
0.220 | ||
| ENC001381 | 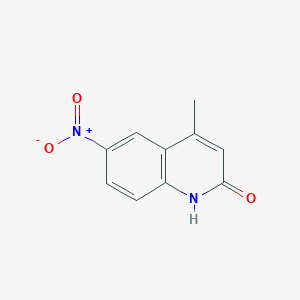 |
0.196 | D0I0DS | 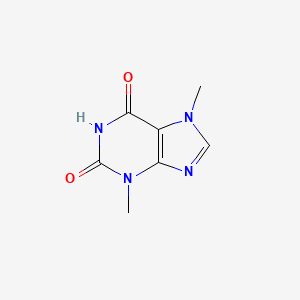 |
0.220 | ||
| ENC000034 | 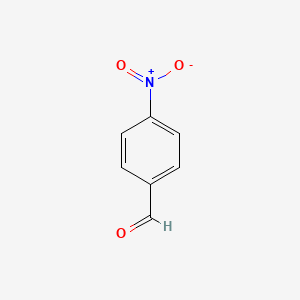 |
0.191 | D07QCE | 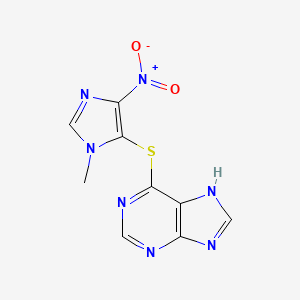 |
0.212 | ||
| ENC000577 | 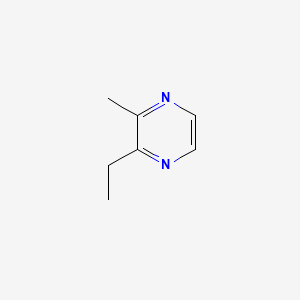 |
0.186 | D0B3HD | 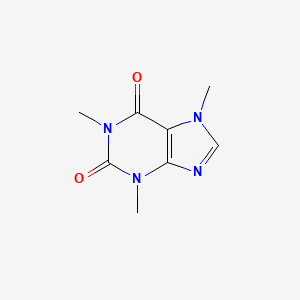 |
0.212 | ||
| ENC001398 | 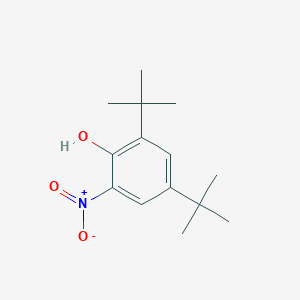 |
0.183 | D0N0OU | 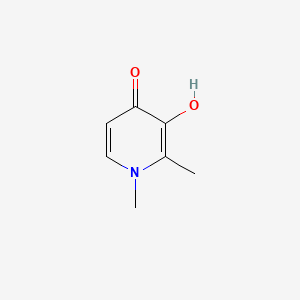 |
0.209 | ||
| ENC002316 | 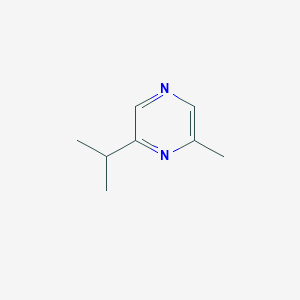 |
0.178 | D0LJ6P | 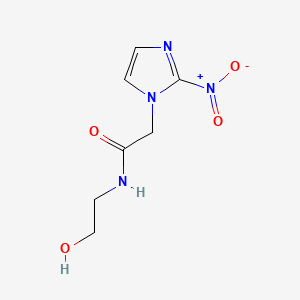 |
0.196 | ||