NPs Basic Information
|
Name |
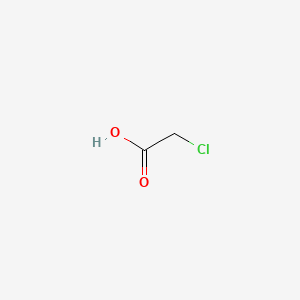
Chloroacetic acid
|
| Molecular Formula | C2H3ClO2 | |
| IUPAC Name* |
2-chloroacetic acid
|
|
| SMILES |
C(C(=O)O)Cl
|
|
| InChI |
InChI=1S/C2H3ClO2/c3-1-2(4)5/h1H2,(H,4,5)
|
|
| InChIKey |
FOCAUTSVDIKZOP-UHFFFAOYSA-N
|
|
| Synonyms |
chloroacetic acid; Monochloroacetic acid; 79-11-8; 2-chloroacetic acid; Chloracetic acid; Chloroethanoic acid; Acetic acid, chloro-; Acide chloracetique; Monochloroethanoic acid; Monochloracetic acid; Monochloorazijnzuur; Monochloressigsaeure; Acidomonocloroacetico; Chloroacetic acid, solid; Chloroacetic; 2-chloro-acetic acid; Acide monochloracetique; Chloroacetic acid [BSI:ISO]; Acetic acid, 2-chloro-; .alpha.-Chloroacetic acid; Acide chloroacetique; Kyselina chloroctova; Monochloorazijnzuur [Dutch]; Acide chloracetique [French]; Kyselina chloroctova [Czech]; Monochloressigsaeure [German]; Acide chloroacetique [French]; alpha-Chloroacetic acid; 2-chloro-ethanoic acid; Acidomonocloroacetico [Italian]; Acide monochloracetique [French]; Chloroacetic acid, molten; Monochloroacetic acid [BSI:ISO]; NSC 142; Acide chloracetique [ISO-French]; Chloroacetic acid (80% or less); NCI-C60231; chloro-acetic acid; Acetocaustin (TN); CH2ClCOOH; monochloro acetic acid; CHLOROACETIC-ACID; Monochloracetic acidacide monochloracetique; Chloroacetic acid, liquid; CHEBI:27869; NSC-142; 5GD84Y125G; MFCD00002683; CHLOROACETIC ACID CRYSTALLINE; Chloroacetic acid, molten [UN3250] [Poison]; Chloroacetic acid, solid [UN1751] [Poison]; Acetocaustin; Caswell No. 179B; sJPhLQDIKTp@; CCRIS 2117; HSDB 939; EINECS 201-178-4; UN1750; UN1751; UN3250; EPA Pesticide Chemical Code 279400; BRN 0605438; CHLOROACETIC ACID, ACS; UNII-5GD84Y125G; cloroacetic acid; AI3-25035; chloro acetic acid; a-chloroacetic acid; R3W; ClCH2COOH; monochloro-acetic acid; alpha-chloro-acetic acid; Chloroacetic acid, 99%; DSSTox_CID_901; Chloroacetic acid, solid [UN1751] [Poison]; bmse000367; WLN: QV1G; Chloroacetic acid, molten [UN3250] [Poison]; EC 201-178-4; Chloroacetic acid, >=99%; DSSTox_RID_75855; NCIOpen2_002217; DSSTox_GSID_20901; 4-02-00-00474 (Beilstein Handbook Reference); MLS001065621; BIDD:ER0630; CHEMBL14090; CHLOROACETIC ACID [MI]; NSC142; CHLOROACETIC ACID [ISO]; Chloroacetic acid, solid (dot); CHLOROACETIC ACID [HSDB]; CHLOROACETIC ACID [INCI]; DTXSID4020901; FOCAUTSVDIKZOP-UHFFFAOYSA-; CHLOROACETIC ACID [VANDF]; BCP20585; NSC42970; STR00326; ZINC3860254; Tox21_201114; BBL037260; LMFA01090068; MONOCHLOROACETIC ACID [VANDF]; NSC-42970; STL197882; Chloroacetic acid, analytical standard; MONOCHLOROACETIC ACID [MART.]; AKOS000118920; MONOCHLOROACETIC ACID [WHO-DD]; UN 1751; CAS-79-11-8; NCGC00091473-01; NCGC00091473-02; NCGC00091473-03; NCGC00258666-01; SMR000568484; Chloroacetic acid, for synthesis, 99.0%; Chloroacetic acid, ACS reagent, >=99.0%; Chloroacetic acid, purum, >=97.0% (T); Chloroacetic acid, puriss., >=99.0% (T); EN300-16767; C06755; D07677; Chloroacetic acid, JIS special grade, >=99.0%; Q409013; J-520023; Chloroacetic acid, PESTANAL(R), analytical standard; F2190-0289; Chloroacetic acid 1000 microg/mL in Methyl-tert-butyl ether
|
|
| CAS | 79-11-8 | |
| PubChem CID | 300 | |
| ChEMBL ID | CHEMBL14090 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 94.5 | ALogp: | 0.2 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 37.3 | Aromatic Rings: | 0 |
| Heavy Atoms: | 5 | QED Weighted: | 0.486 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.653 | MDCK Permeability: | 0.00126743 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.002 |
| 30% Bioavailability (F30%): | 0.006 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.842 | Plasma Protein Binding (PPB): | 33.91% |
| Volume Distribution (VD): | 0.254 | Fu: | 67.81% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.021 | CYP1A2-substrate: | 0.114 |
| CYP2C19-inhibitor: | 0.028 | CYP2C19-substrate: | 0.064 |
| CYP2C9-inhibitor: | 0.007 | CYP2C9-substrate: | 0.906 |
| CYP2D6-inhibitor: | 0.012 | CYP2D6-substrate: | 0.237 |
| CYP3A4-inhibitor: | 0.008 | CYP3A4-substrate: | 0.045 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.903 | Half-life (T1/2): | 0.901 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.113 | Human Hepatotoxicity (H-HT): | 0.052 |
| Drug-inuced Liver Injury (DILI): | 0.192 | AMES Toxicity: | 0.441 |
| Rat Oral Acute Toxicity: | 0.965 | Maximum Recommended Daily Dose: | 0.012 |
| Skin Sensitization: | 0.853 | Carcinogencity: | 0.304 |
| Eye Corrosion: | 0.997 | Eye Irritation: | 0.989 |
| Respiratory Toxicity: | 0.983 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000058 | 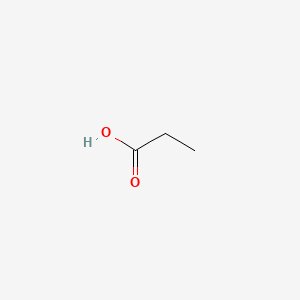 |
0.467 | D0M8AB | 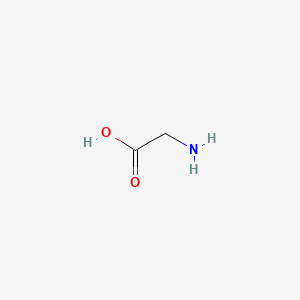 |
0.467 | ||
| ENC000677 | 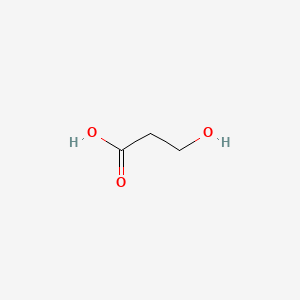 |
0.368 | D04CRL | 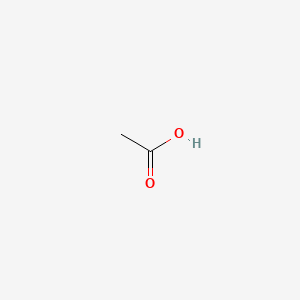 |
0.357 | ||
| ENC000018 | 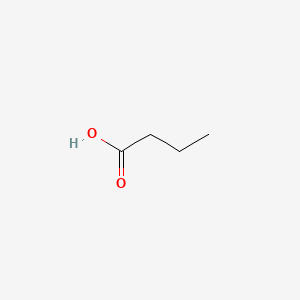 |
0.368 | D02FLB |  |
0.333 | ||
| ENC000009 | 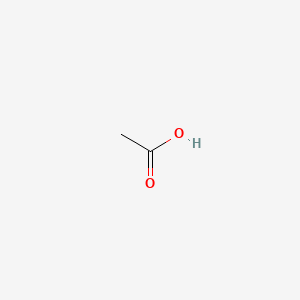 |
0.357 | D0EP8X | 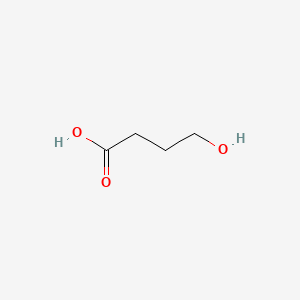 |
0.318 | ||
| ENC000351 | 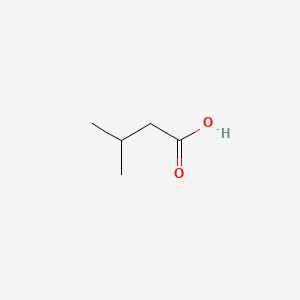 |
0.333 | D02KJX | 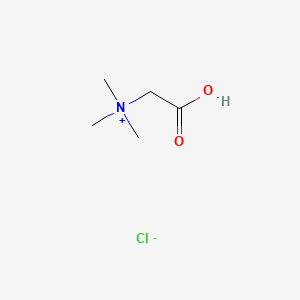 |
0.292 | ||
| ENC000031 | 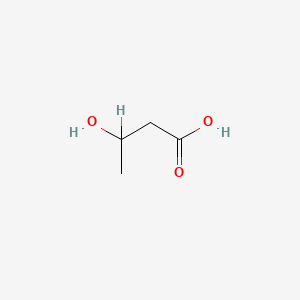 |
0.333 | D06VNK | 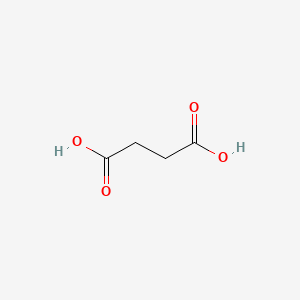 |
0.292 | ||
| ENC000639 | 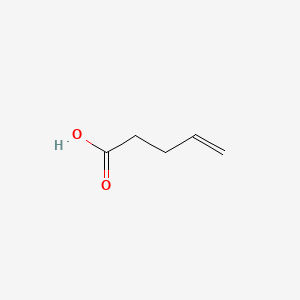 |
0.318 | D02UDJ | 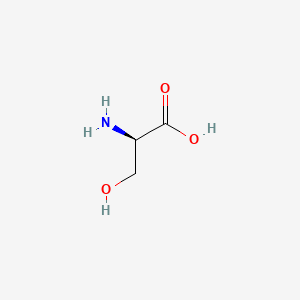 |
0.273 | ||
| ENC000288 | 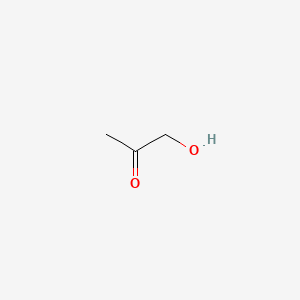 |
0.294 | D0G4JI | 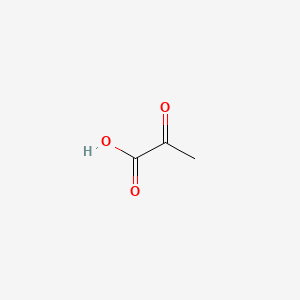 |
0.263 | ||
| ENC000148 | 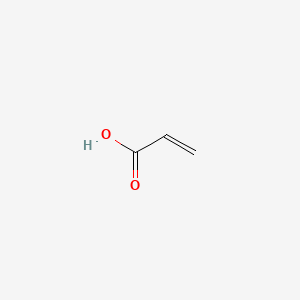 |
0.294 | D09PUL | 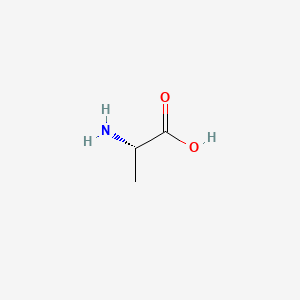 |
0.263 | ||
| ENC000445 | 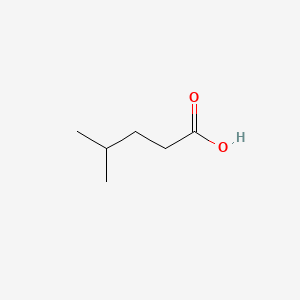 |
0.292 | D0P0QK | 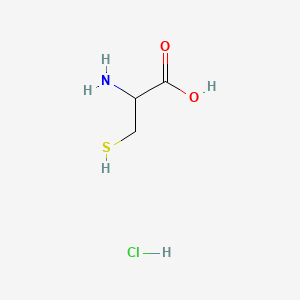 |
0.261 | ||