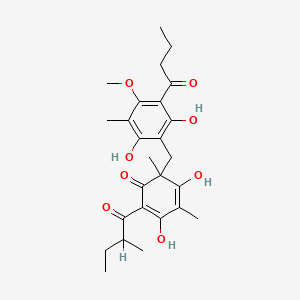
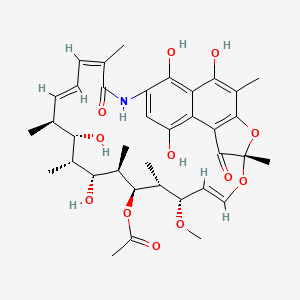
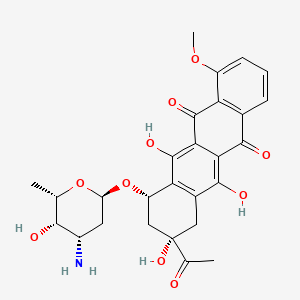
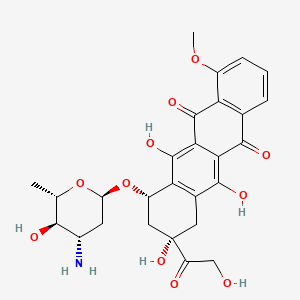
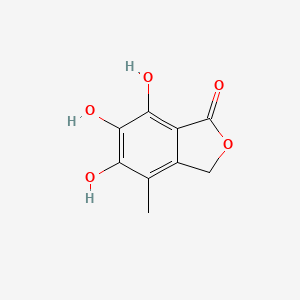
NPs Basic Information

|
Name |
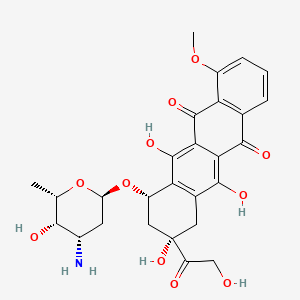
Aspermicrone B
|
| Molecular Formula | C19H18O10 | |
| IUPAC Name* |
(1R,3R)-4,5,5',6,6',7'-hexahydroxy-1-methoxy-7,8'-dimethylspiro[1H-2-benzofuran-3,3'-1H-isochromene]-4'-one
|
|
| SMILES |
CC1=C2CO[C@@]3(C4=C(C(=C(C(=C4[C@@H](O3)OC)C)O)O)O)C(=O)C2=C(C(=C1O)O)O
|
|
| InChI |
InChI=1S/C19H18O10/c1-5-7-4-28-19(17(26)9(7)13(22)15(24)11(5)20)10-8(18(27-3)29-19)6(2)12(21)16(25)14(10)23/h18,20-25H,4H2,1-3H3/t18-,19-/m1/s1
|
|
| InChIKey |
QMVOBCWDKWKDII-RTBURBONSA-N
|
|
| Synonyms |
Aspermicrone B
|
|
| CAS | NA | |
| PubChem CID | 145720716 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Physi-Chem Properties
| Molecular Weight: | 406.3 | ALogp: | 1.3 |
| HBD: | 6 | HBA: | 10 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 166.0 | Aromatic Rings: | 4 |
| Heavy Atoms: | 29 | QED Weighted: | 0.387 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.969 | MDCK Permeability: | 0.00000413 |
| Pgp-inhibitor: | 0.05 | Pgp-substrate: | 0.469 |
| Human Intestinal Absorption (HIA): | 0.889 | 20% Bioavailability (F20%): | 0.656 |
| 30% Bioavailability (F30%): | 0.966 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.006 | Plasma Protein Binding (PPB): | 98.61% |
| Volume Distribution (VD): | 0.495 | Fu: | 4.04% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.033 | CYP1A2-substrate: | 0.918 |
| CYP2C19-inhibitor: | 0.01 | CYP2C19-substrate: | 0.071 |
| CYP2C9-inhibitor: | 0.066 | CYP2C9-substrate: | 0.131 |
| CYP2D6-inhibitor: | 0.004 | CYP2D6-substrate: | 0.141 |
| CYP3A4-inhibitor: | 0.031 | CYP3A4-substrate: | 0.15 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 1.04 | Half-life (T1/2): | 0.863 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.029 | Human Hepatotoxicity (H-HT): | 0.226 |
| Drug-inuced Liver Injury (DILI): | 0.982 | AMES Toxicity: | 0.524 |
| Rat Oral Acute Toxicity: | 0.039 | Maximum Recommended Daily Dose: | 0.482 |
| Skin Sensitization: | 0.961 | Carcinogencity: | 0.516 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.91 |
| Respiratory Toxicity: | 0.039 |