NPs Basic Information
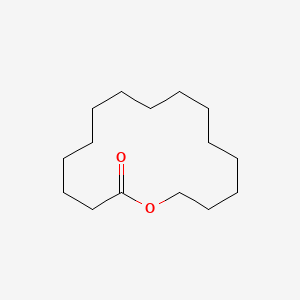
|
Name |
Oxacyclohexadecan-2-one
|
| Molecular Formula | C15H28O2 | |
| IUPAC Name* |
oxacyclohexadecan-2-one
|
|
| SMILES |
C1CCCCCCCOC(=O)CCCCCC1
|
|
| InChI |
InChI=1S/C15H28O2/c16-15-13-11-9-7-5-3-1-2-4-6-8-10-12-14-17-15/h1-14H2
|
|
| InChIKey |
FKUPPRZPSYCDRS-UHFFFAOYSA-N
|
|
| Synonyms |
Oxacyclohexadecan-2-one; 106-02-5; Cyclopentadecanolide; Pentadecanolide; Exaltolide; Pentalide; Pentadecan-15-olide; 15-Pentadecanolide; PENTADECALACTONE; Muskolactone; Thibetolide; 15-Pentadecanolactone; Muskalactone; 2-Pentadecalone; omega-Pentadecalactone; 1,15-Pentadecanolide; Pentadecanolactone; 1-oxacyclohexadecan-2-one; 1-Oxa-2-cyclohexadecanone; 15-Hydroxypentadecanoic acid lactone; FEMA No. 2840; Cyclopentadecanolactone; pentadecano-15-lactone; NSC 36763; 15-Hydroxypentadecanoic acid, lactone; OK17S3S98K; Pentadecanoic acid, 15-hydroxy-, .xi.-lactone; NSC-36763; 36486-90-5; UNII-OK17S3S98K; CCRIS 3682; cyclopentadecalactone; EINECS 203-354-6; MFCD00039667; EXALTEX; 14-Oxytetradecane carbonic acid lactone; Pentadecanolide, 98%; .OMEGA.-LACTONE; 1,15 -pentadecanolide; EXALTOLIDE [MI]; 1, 15-Pentadecanolide; AI3-30956; Pentadecanoic acid, 15-hydroxy-, xi-lactone; EC 203-354-6; DSSTox_CID_24359; DSSTox_RID_80167; DSSTox_GSID_44359; PENTADECALACTONE [II]; SCHEMBL114310; PENTADECALACTONE [INCI]; .OMEGA.-PENTADECALACTONE; CHEMBL3560504; DTXSID6044359; FEMA 2840; CPE-215; Pentadecanoic acid, .xi.-lactone; 15-Hydroxypentadecanoicacidlactone; CHEBI:177420; NSC36763; Pentadecanolide, analytical standard; ZINC4023520; OMEGA-PENTADECALACTONE [FCC]; Tox21_301123; Oxacyclohexadecan-2-one, 9CI, 8CI; STL560823; 15-HYDROXYPENTADECA-NOIC ACID; omega-Pentadecalactone, >=98%, FG; AKOS015964064; AC-9864; HY-W035362; .OMEGA.-PENTADECALACTONE [FHFI]; NCGC00248297-01; NCGC00255022-01; AS-57924; CAS-106-02-5; DB-040675; 15-Hydroxypentadecanoic acid-epsilon-lactone; CS-0085751; FT-0624246; P0985; Pentadecanolide, puriss., >=99.0% (GC); EN300-254580; F20373; A801360; J-001527; Q9251595; Z57959539; Pentadecanoic acid, 15-hydroxy-, laquo Xiraquo -lactone
|
|
| CAS | 106-02-5 | |
| PubChem CID | 235414 | |
| ChEMBL ID | CHEMBL3560504 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 240.38 | ALogp: | 5.8 |
| HBD: | 0 | HBA: | 2 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 26.3 | Aromatic Rings: | 1 |
| Heavy Atoms: | 17 | QED Weighted: | 0.552 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.847 | MDCK Permeability: | 0.00002550 |
| Pgp-inhibitor: | 0.058 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.917 |
| 30% Bioavailability (F30%): | 0.998 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.338 | Plasma Protein Binding (PPB): | 96.72% |
| Volume Distribution (VD): | 1.234 | Fu: | 2.04% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.876 | CYP1A2-substrate: | 0.188 |
| CYP2C19-inhibitor: | 0.588 | CYP2C19-substrate: | 0.06 |
| CYP2C9-inhibitor: | 0.299 | CYP2C9-substrate: | 0.905 |
| CYP2D6-inhibitor: | 0.068 | CYP2D6-substrate: | 0.084 |
| CYP3A4-inhibitor: | 0.336 | CYP3A4-substrate: | 0.075 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 5.987 | Half-life (T1/2): | 0.383 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.255 | Human Hepatotoxicity (H-HT): | 0.025 |
| Drug-inuced Liver Injury (DILI): | 0.3 | AMES Toxicity: | 0.01 |
| Rat Oral Acute Toxicity: | 0.038 | Maximum Recommended Daily Dose: | 0.025 |
| Skin Sensitization: | 0.958 | Carcinogencity: | 0.156 |
| Eye Corrosion: | 0.963 | Eye Irritation: | 0.98 |
| Respiratory Toxicity: | 0.84 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000324 |  |
0.654 | D00SBN |  |
0.279 | ||
| ENC000893 |  |
0.648 | D0S5NG | 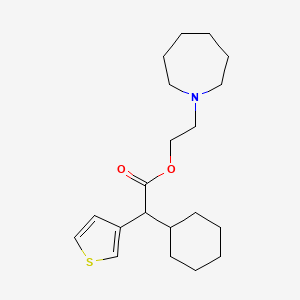 |
0.235 | ||
| ENC000323 | 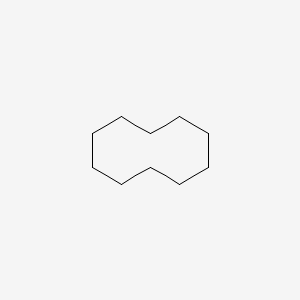 |
0.600 | D08VSI | 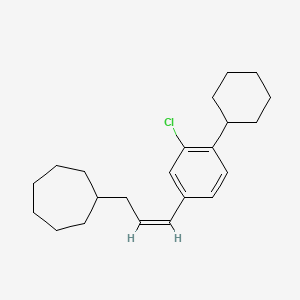 |
0.229 | ||
| ENC000840 | 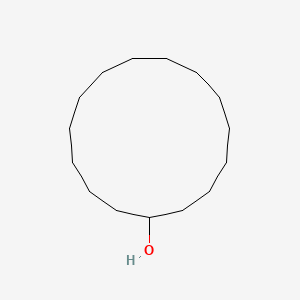 |
0.590 | D0Z8AA |  |
0.216 | ||
| ENC001146 |  |
0.486 | D07XJM |  |
0.202 | ||
| ENC001147 |  |
0.419 | D0N3PE | 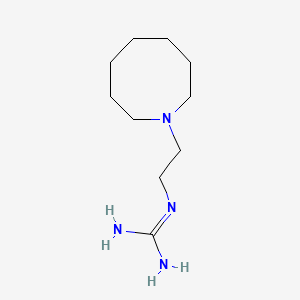 |
0.200 | ||
| ENC005597 | 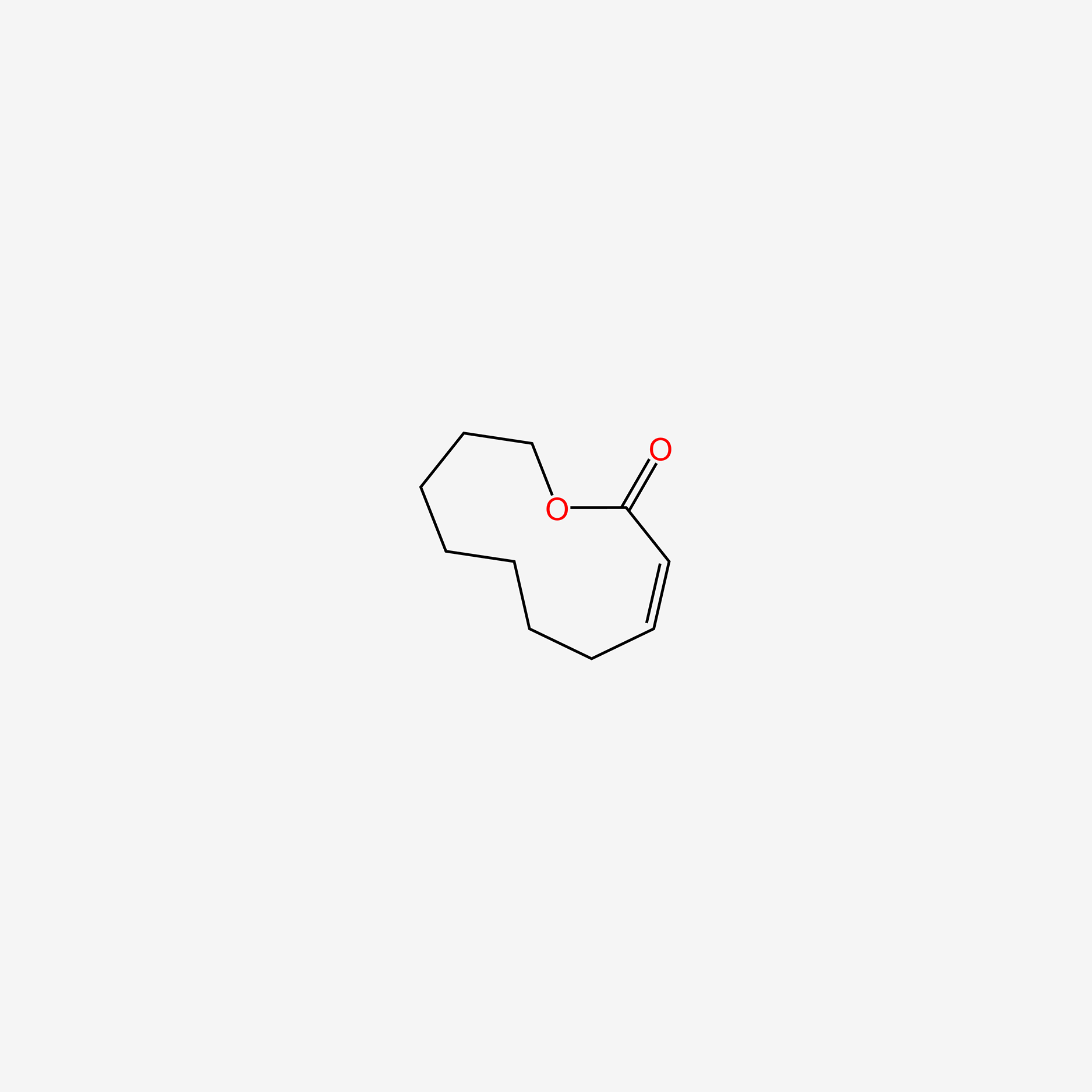 |
0.371 | D09GFL | 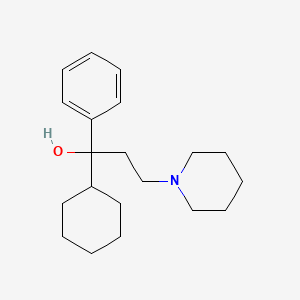 |
0.198 | ||
| ENC000251 | 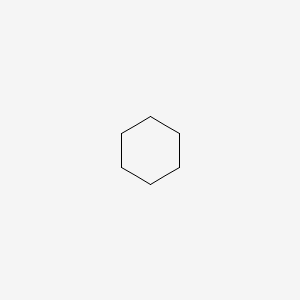 |
0.360 | D0U3CR | 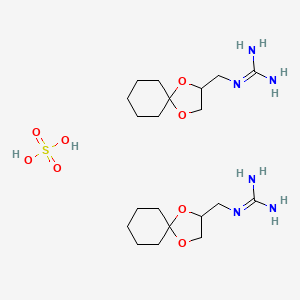 |
0.177 | ||
| ENC001230 | 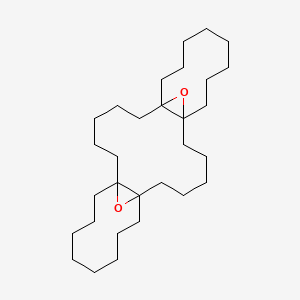 |
0.339 | D0L0MK | 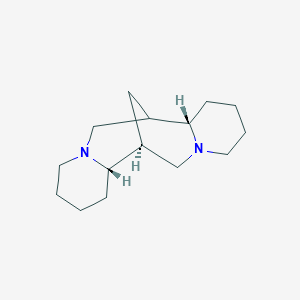 |
0.174 | ||
| ENC005710 | 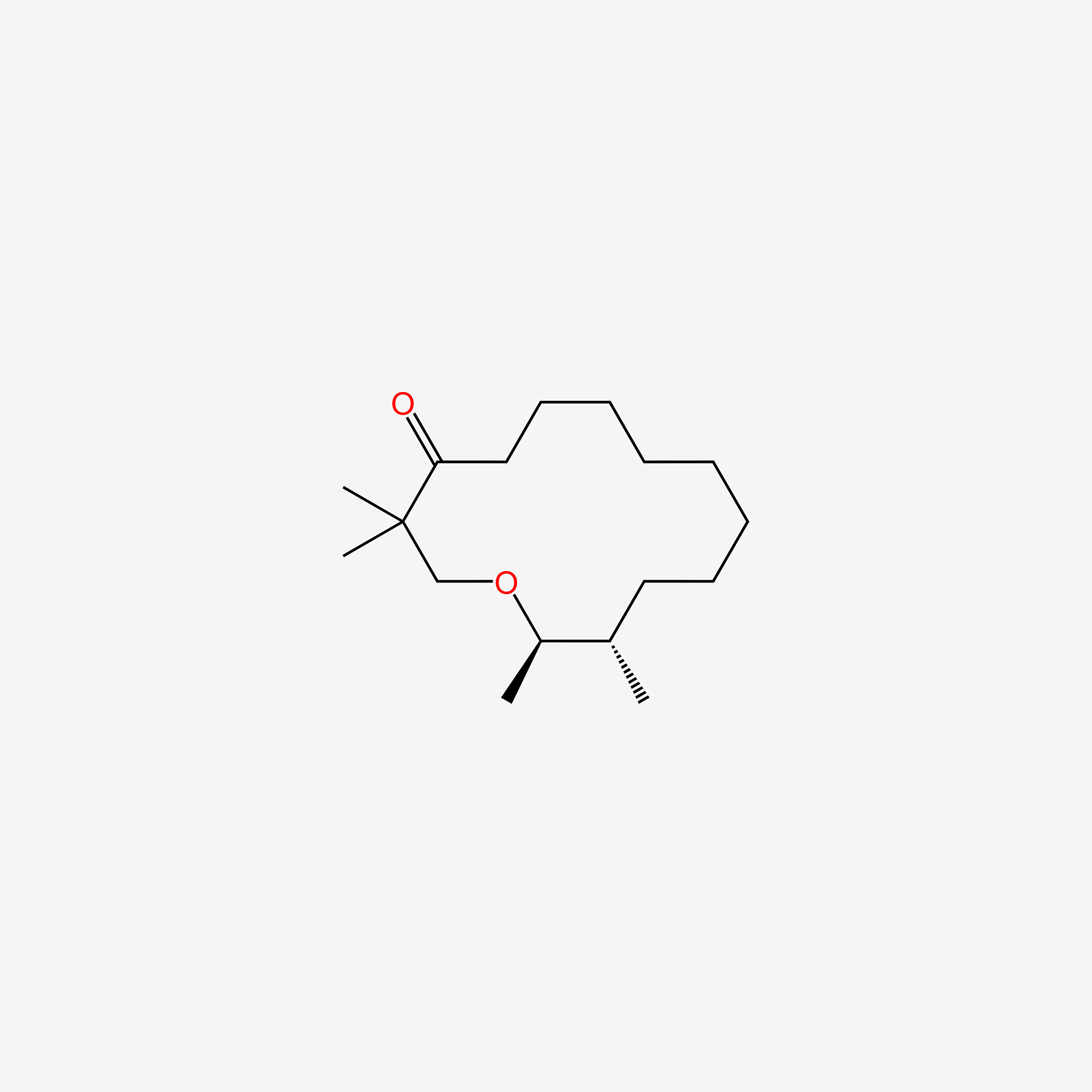 |
0.325 | D0L9ZR | 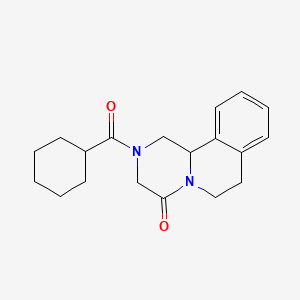 |
0.170 | ||