NPs Basic Information
|
Name |
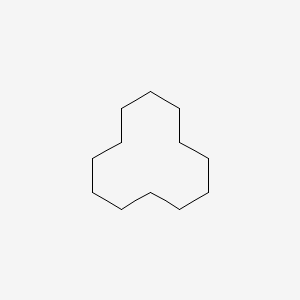
Cyclododecane
|
| Molecular Formula | C12H24 | |
| IUPAC Name* |
cyclododecane
|
|
| SMILES |
C1CCCCCCCCCCC1
|
|
| InChI |
InChI=1S/C12H24/c1-2-4-6-8-10-12-11-9-7-5-3-1/h1-12H2
|
|
| InChIKey |
DDTBPAQBQHZRDW-UHFFFAOYSA-N
|
|
| Synonyms |
CYCLODODECANE; 294-62-2; Cyclododecan; 97CN13ZD83; EINECS 206-033-9; HSDB 5557; BRN 1901008; UNII-97CN13ZD83; DSSTox_CID_1552; EC 206-033-9; CYCLODODECANE [HSDB]; DSSTox_RID_76206; DSSTox_GSID_21552; 4-05-00-00169 (Beilstein Handbook Reference); CHEMBL3185808; DTXSID9021552; Tox21_202734; MFCD00014258; ZINC54962163; AKOS006227986; NCGC00260282-01; AS-56381; CAS-294-62-2; DB-047586; CS-0155296; FT-0624163; D89269; Q118040
|
|
| CAS | 294-62-2 | |
| PubChem CID | 9268 | |
| ChEMBL ID | CHEMBL3185808 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 168.32 | ALogp: | 6.7 |
| HBD: | 0 | HBA: | 0 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 0.0 | Aromatic Rings: | 1 |
| Heavy Atoms: | 12 | QED Weighted: | 0.471 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.773 | MDCK Permeability: | 0.00001610 |
| Pgp-inhibitor: | 0.002 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.27 |
| 30% Bioavailability (F30%): | 0.992 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.515 | Plasma Protein Binding (PPB): | 97.48% |
| Volume Distribution (VD): | 3.22 | Fu: | 1.74% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.7 | CYP1A2-substrate: | 0.203 |
| CYP2C19-inhibitor: | 0.494 | CYP2C19-substrate: | 0.067 |
| CYP2C9-inhibitor: | 0.149 | CYP2C9-substrate: | 0.94 |
| CYP2D6-inhibitor: | 0.112 | CYP2D6-substrate: | 0.111 |
| CYP3A4-inhibitor: | 0.138 | CYP3A4-substrate: | 0.058 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 3.944 | Half-life (T1/2): | 0.122 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.162 | Human Hepatotoxicity (H-HT): | 0.021 |
| Drug-inuced Liver Injury (DILI): | 0.353 | AMES Toxicity: | 0.027 |
| Rat Oral Acute Toxicity: | 0.044 | Maximum Recommended Daily Dose: | 0.043 |
| Skin Sensitization: | 0.955 | Carcinogencity: | 0.075 |
| Eye Corrosion: | 0.992 | Eye Irritation: | 0.971 |
| Respiratory Toxicity: | 0.869 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000893 | 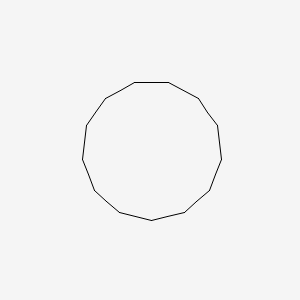 |
0.923 | D00SBN | 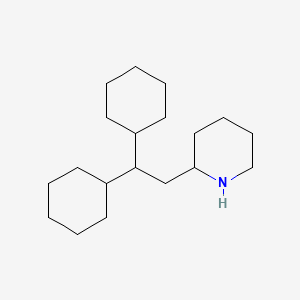 |
0.297 | ||
| ENC000323 | 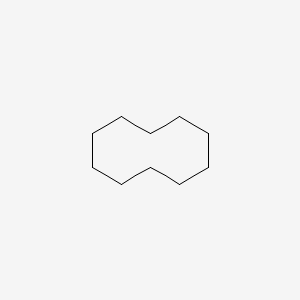 |
0.833 | D08VSI | 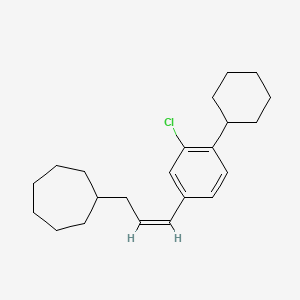 |
0.253 | ||
| ENC000840 | 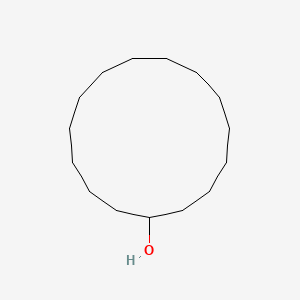 |
0.694 | D0N3PE | 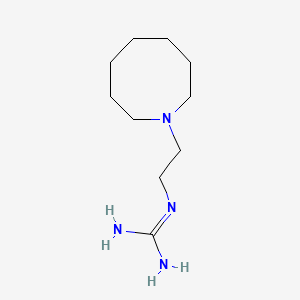 |
0.246 | ||
| ENC001017 | 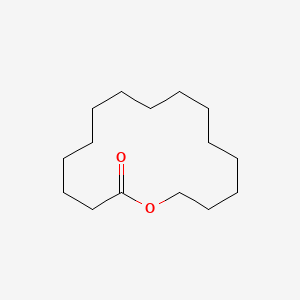 |
0.654 | D0S5NG | 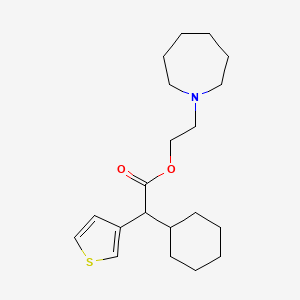 |
0.244 | ||
| ENC001146 | 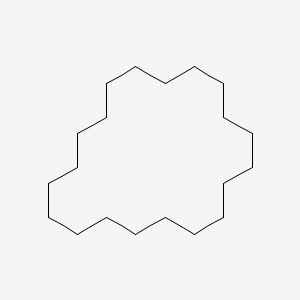 |
0.600 | D07XJM | 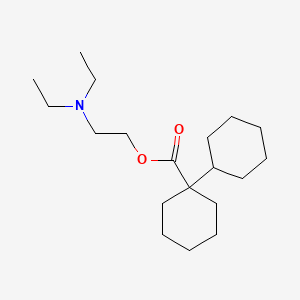 |
0.222 | ||
| ENC000251 | 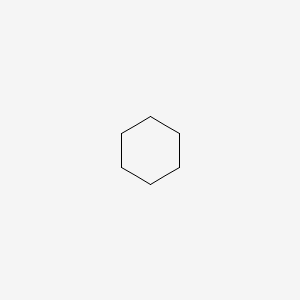 |
0.500 | D09GFL | 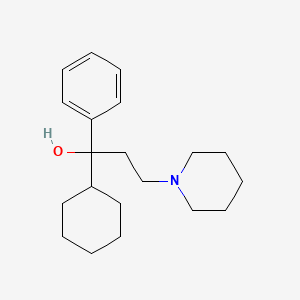 |
0.217 | ||
| ENC001147 | 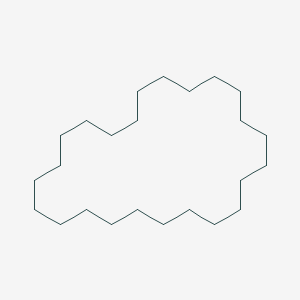 |
0.500 | D0L0MK | 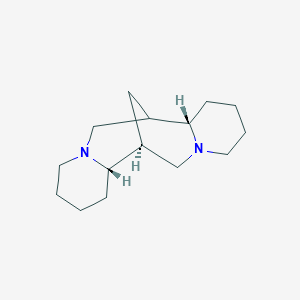 |
0.208 | ||
| ENC001230 | 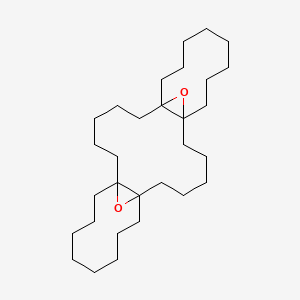 |
0.347 | D02LRQ | 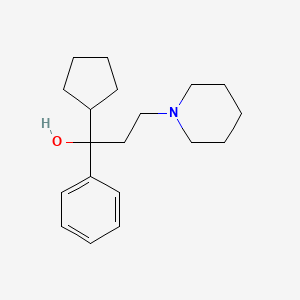 |
0.181 | ||
| ENC000170 | 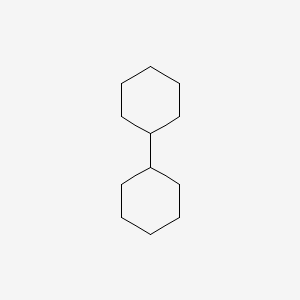 |
0.333 | D0R1WR | 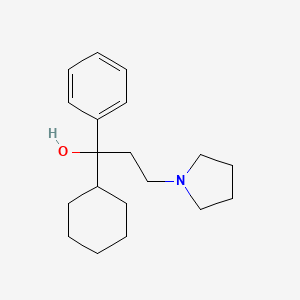 |
0.181 | ||
| ENC005710 | 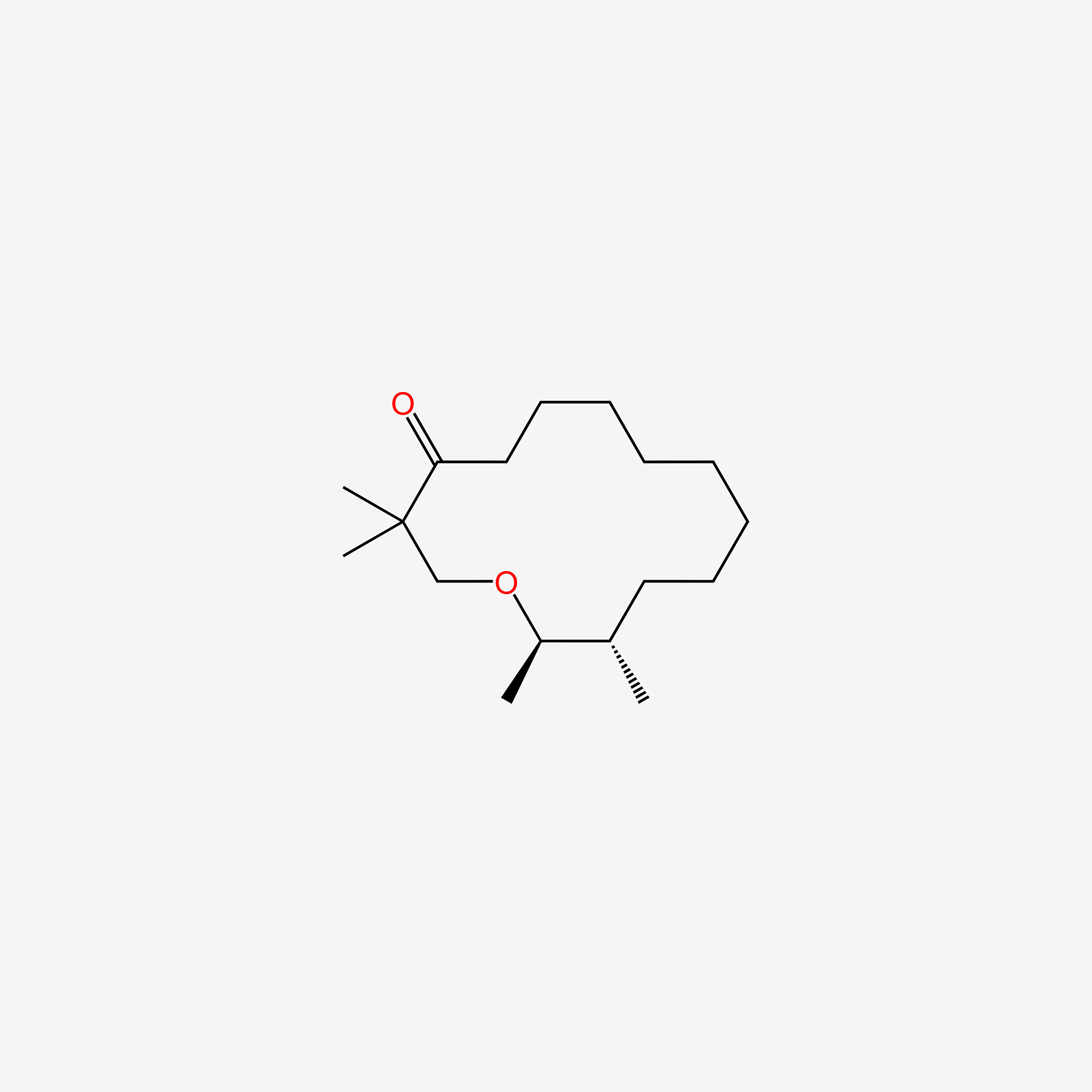 |
0.275 | D0U3CR | 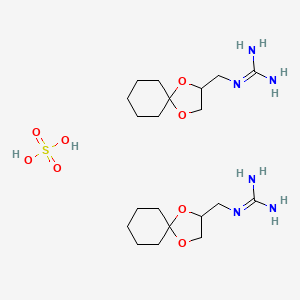 |
0.179 | ||