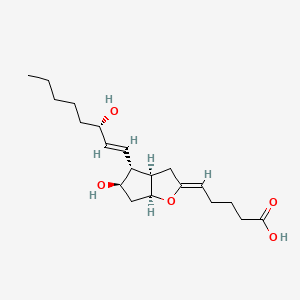
NPs Basic Information

|
Name |
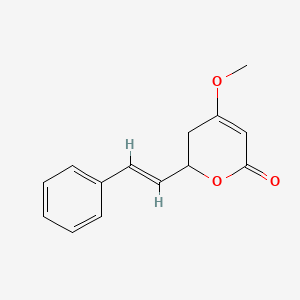
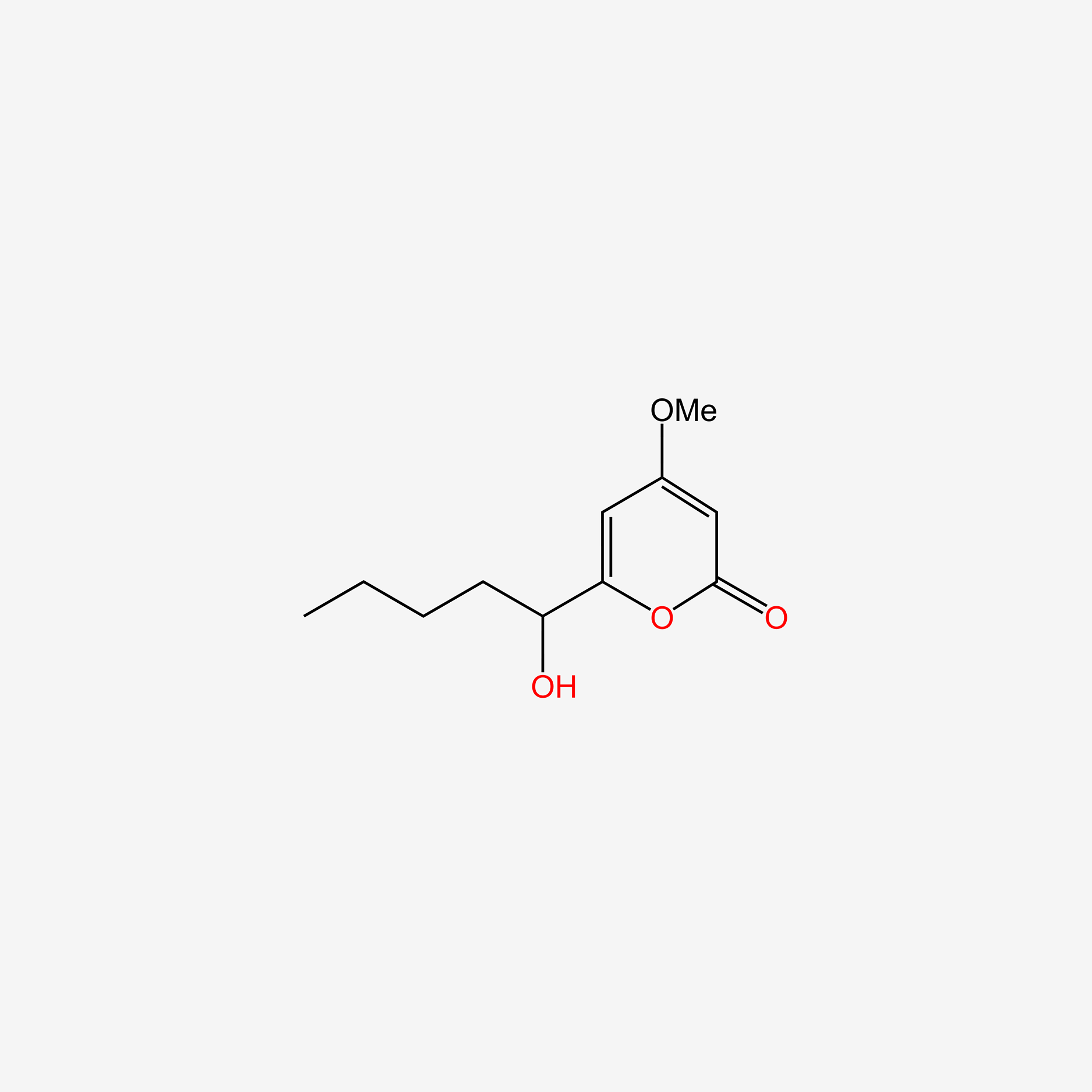
Pestalotin
|
| Molecular Formula | C11H18O4 | |
| IUPAC Name* |
(2S)-2-[(1S)-1-hydroxypentyl]-4-methoxy-2,3-dihydropyran-6-one
|
|
| SMILES |
CCCC[C@@H]([C@@H]1CC(=CC(=O)O1)OC)O
|
|
| InChI |
InChI=1S/C11H18O4/c1-3-4-5-9(12)10-6-8(14-2)7-11(13)15-10/h7,9-10,12H,3-6H2,1-2H3/t9-,10-/m0/s1
|
|
| InChIKey |
YFIMUDXPJZVJJO-UWVGGRQHSA-N
|
|
| Synonyms |
Pestalotin; 34565-32-7; (+/-)-Pestalotin; (2S)-2-[(1S)-1-hydroxypentyl]-4-methoxy-2,3-dihydropyran-6-one; (-)-pestalotin; C11H18O4; (2S)-2-((1S)-1-Hydroxypentyl)-4-methoxy-2,3-dihydropyran-6-one; 6-(1-hydroxypentyl)-4-methoxy-5,6-dihydro-2H-pyran-2-one; ACon1_001546; DTXSID20956075; CHEBI:182798; ZINC5821894; (6S)-6-[(1S)-1-Hydroxypentyl]-4-methoxy-5,6-dihydro-2H-pyran-2-one; NCGC00180394-01; HY-119328; CS-0067052; BRD-K86217670-001-01-9; NCGC00180394-02!(2S)-2-[(1S)-1-hydroxypentyl]-4-methoxy-2,3-dihydropyran-6-one
|
|
| CAS | 34565-32-7 | |
| PubChem CID | 182201 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Physi-Chem Properties
| Molecular Weight: | 214.26 | ALogp: | 1.6 |
| HBD: | 1 | HBA: | 4 |
| Rotatable Bonds: | 5 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 55.8 | Aromatic Rings: | 1 |
| Heavy Atoms: | 15 | QED Weighted: | 0.71 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.55 | MDCK Permeability: | 0.00004520 |
| Pgp-inhibitor: | 0.152 | Pgp-substrate: | 0.098 |
| Human Intestinal Absorption (HIA): | 0.022 | 20% Bioavailability (F20%): | 0.161 |
| 30% Bioavailability (F30%): | 0.979 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.735 | Plasma Protein Binding (PPB): | 34.39% |
| Volume Distribution (VD): | 0.635 | Fu: | 52.02% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.133 | CYP1A2-substrate: | 0.301 |
| CYP2C19-inhibitor: | 0.088 | CYP2C19-substrate: | 0.836 |
| CYP2C9-inhibitor: | 0.045 | CYP2C9-substrate: | 0.229 |
| CYP2D6-inhibitor: | 0.007 | CYP2D6-substrate: | 0.304 |
| CYP3A4-inhibitor: | 0.074 | CYP3A4-substrate: | 0.305 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 12.753 | Half-life (T1/2): | 0.864 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.03 | Human Hepatotoxicity (H-HT): | 0.322 |
| Drug-inuced Liver Injury (DILI): | 0.206 | AMES Toxicity: | 0.076 |
| Rat Oral Acute Toxicity: | 0.556 | Maximum Recommended Daily Dose: | 0.822 |
| Skin Sensitization: | 0.966 | Carcinogencity: | 0.499 |
| Eye Corrosion: | 0.902 | Eye Irritation: | 0.954 |
| Respiratory Toxicity: | 0.768 |