NPs Basic Information
|
Name |
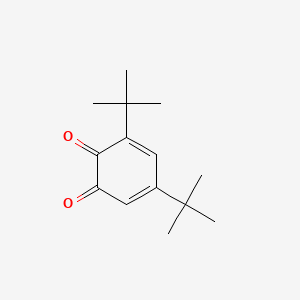
3,5-Di-tert-butyl-o-benzoquinone
|
| Molecular Formula | C14H20O2 | |
| IUPAC Name* |
3,5-ditert-butylcyclohexa-3,5-diene-1,2-dione
|
|
| SMILES |
CC(C)(C)C1=CC(=O)C(=O)C(=C1)C(C)(C)C
|
|
| InChI |
InChI=1S/C14H20O2/c1-13(2,3)9-7-10(14(4,5)6)12(16)11(15)8-9/h7-8H,1-6H3
|
|
| InChIKey |
NOUZOVBGCDDMSX-UHFFFAOYSA-N
|
|
| Synonyms |
3,5-Di-tert-butyl-o-benzoquinone; 3383-21-9; 3,5-Di-tert-butyl-1,2-benzoquinone; 3,5-di(tert-butyl)benzo-1,2-quinone; 3,5-Cyclohexadiene-1,2-dione, 3,5-bis(1,1-dimethylethyl)-; 3,5-di-tert-butylbenzo-1,2-quinone; 3,5-di-tert-butylcyclohexa-3,5-diene-1,2-dione; o-Benzoquinone, 3,5-di-tert-butyl-; 3,5-ditert-butylcyclohexa-3,5-diene-1,2-dione; 3,5-Di-tert-butyl-o-quinone; 3,5-di-t-Butyl-o-benzoquinone; 3,5-di-t-butyl-1,2-benzoquinone; 3,5-Ditert-butylbenzo-1,2-quinone; EINECS 222-189-0; MFCD00001647; NSC 149061; 3,5-Ditert-butyl-quinone; cid_76915; MLS000699499; SCHEMBL396841; CHEMBL365161; BDBM22775; DTXSID70187478; o-Benzoquinone,5-di-tert-butyl-; HMS2618F24; 3,5-di-t-butyl-1,2-dioxobenzene; ALBB-029895; ZINC1734421; NSC149061; 3,5-ditert-butyl-[1,2]benzoquinone; 3,5-di-tert-Butyl-ortho-benzoquinone; AKOS001667084; NSC-149061; 3,5-Ditert-butylbenzo-1,2-quinone #; NCGC00247199-01; 3,5-Di-tert-butyl-o-benzoquinone, 98%; AS-60966; SMR000226412; 3,2-dione, 3,5-bis(1,1-dimethylethyl)-; CS-0095759; D2430; FT-0614738; 3,5-Bis(tert-butyl)-1,2-benzoquinone 98%; D86498; AH-188/25003063; J-511242
|
|
| CAS | 3383-21-9 | |
| PubChem CID | 76915 | |
| ChEMBL ID | CHEMBL365161 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 220.31 | ALogp: | 3.3 |
| HBD: | 0 | HBA: | 2 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 34.1 | Aromatic Rings: | 1 |
| Heavy Atoms: | 16 | QED Weighted: | 0.458 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.832 | MDCK Permeability: | 0.00001580 |
| Pgp-inhibitor: | 0.995 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.014 | 20% Bioavailability (F20%): | 0.435 |
| 30% Bioavailability (F30%): | 0.042 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.012 | Plasma Protein Binding (PPB): | 94.17% |
| Volume Distribution (VD): | 1.818 | Fu: | 6.99% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.92 | CYP1A2-substrate: | 0.953 |
| CYP2C19-inhibitor: | 0.917 | CYP2C19-substrate: | 0.845 |
| CYP2C9-inhibitor: | 0.803 | CYP2C9-substrate: | 0.798 |
| CYP2D6-inhibitor: | 0.706 | CYP2D6-substrate: | 0.274 |
| CYP3A4-inhibitor: | 0.274 | CYP3A4-substrate: | 0.386 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 3.51 | Half-life (T1/2): | 0.334 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.012 | Human Hepatotoxicity (H-HT): | 0.209 |
| Drug-inuced Liver Injury (DILI): | 0.244 | AMES Toxicity: | 0.104 |
| Rat Oral Acute Toxicity: | 0.629 | Maximum Recommended Daily Dose: | 0.839 |
| Skin Sensitization: | 0.906 | Carcinogencity: | 0.794 |
| Eye Corrosion: | 0.947 | Eye Irritation: | 0.96 |
| Respiratory Toxicity: | 0.55 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000452 | 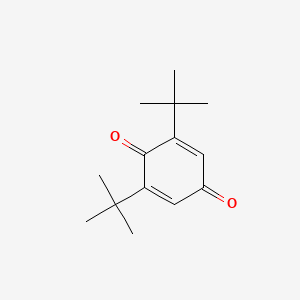 |
0.667 | D0W7WC | 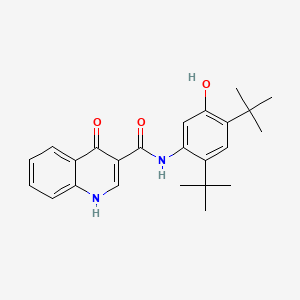 |
0.269 | ||
| ENC000513 | 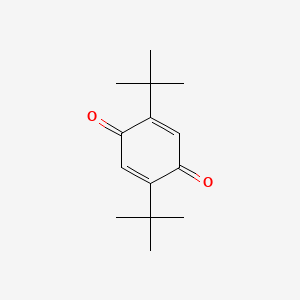 |
0.633 | D01JFT | 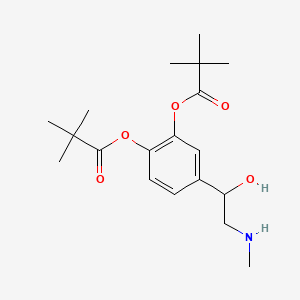 |
0.250 | ||
| ENC000811 | 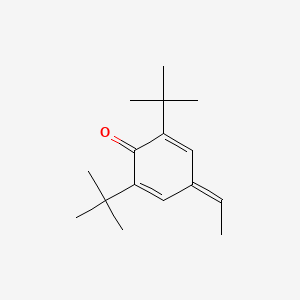 |
0.566 | D03GET | 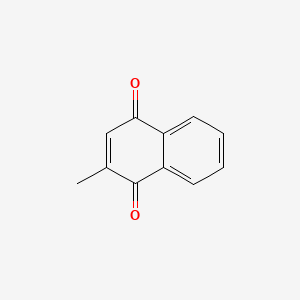 |
0.226 | ||
| ENC001383 | 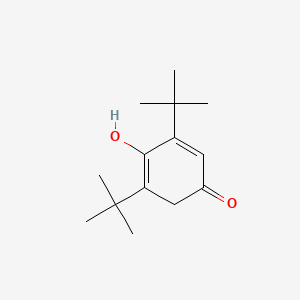 |
0.481 | D0Y4DY | 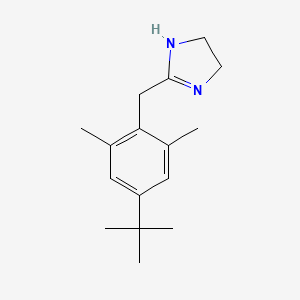 |
0.219 | ||
| ENC001233 | 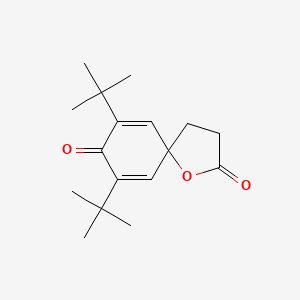 |
0.460 | D06YPU | 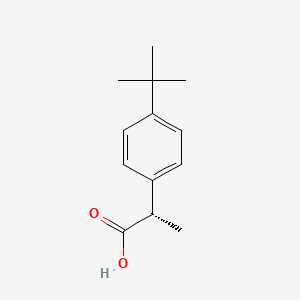 |
0.215 | ||
| ENC000708 | 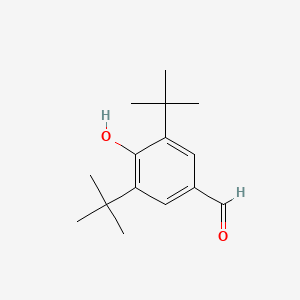 |
0.383 | D00NJL | 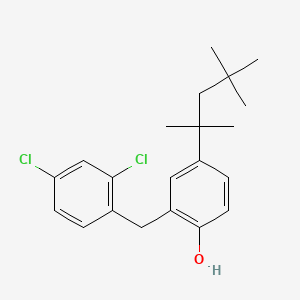 |
0.209 | ||
| ENC000610 |  |
0.379 | D0H2DQ | 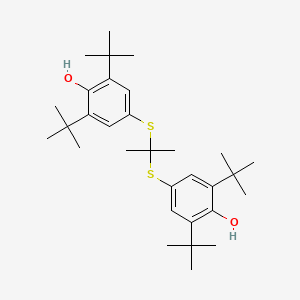 |
0.206 | ||
| ENC000079 | 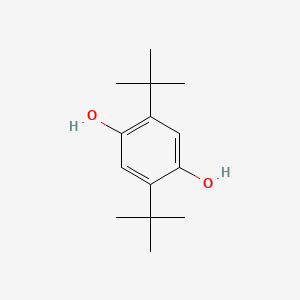 |
0.379 | D09EBS | 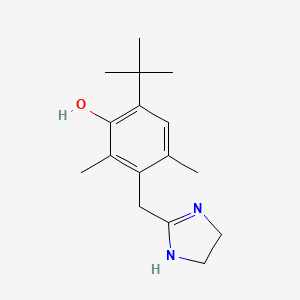 |
0.197 | ||
| ENC000346 | 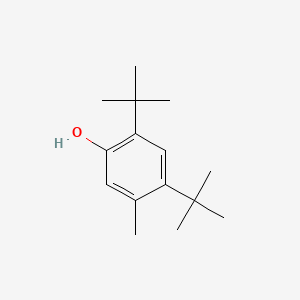 |
0.379 | D0ML1F | 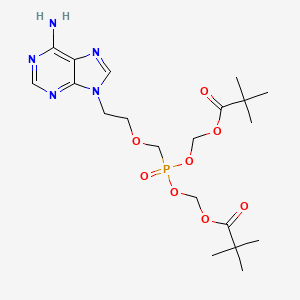 |
0.189 | ||
| ENC000725 | 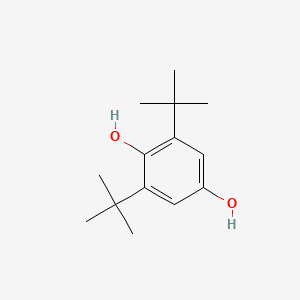 |
0.379 | D0X4ZR | 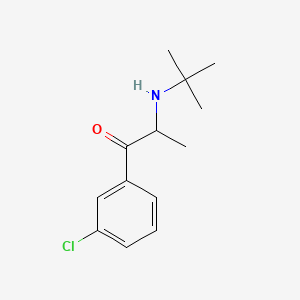 |
0.188 | ||