NPs Basic Information
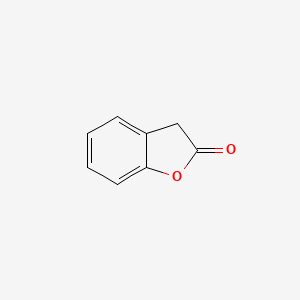
|
Name |
2-Coumaranone
|
| Molecular Formula | C8H6O2 | |
| IUPAC Name* |
3H-1-benzofuran-2-one
|
|
| SMILES |
C1C2=CC=CC=C2OC1=O
|
|
| InChI |
InChI=1S/C8H6O2/c9-8-5-6-3-1-2-4-7(6)10-8/h1-4H,5H2
|
|
| InChIKey |
ACZGCWSMSTYWDQ-UHFFFAOYSA-N
|
|
| Synonyms |
2-Coumaranone; 553-86-6; Benzofuran-2(3H)-one; 2(3H)-Benzofuranone; 3H-benzofuran-2-one; Benzofuran-2-one; Benzofuranone; (3H)-Benzofuran-2-one; Isophthalide; 1-Benzofuran-2(3H)-one; 3H-1-benzofuran-2-one; 2-Coumarotioiie; MFCD00005856; CHEMBL284584; 4K47Z4Q1E7; NSC-227414; Isocoumaranone; 2,3-dihydrobenzofuran-2-one; UNII-4K47Z4Q1E7; 2-Benzofuranone; 2,3-dihydro-1-benzofuran-2-one; EINECS 209-052-0; NSC227414; CUMARANONE; 2-COUMARONE; 2-Coumaranone, 97%; AI3-36067; EC 209-052-0; SCHEMBL44758; 2 (3H) - benzo-furanone; 2,3-dihydro-2-benzofuranone; 1-Benzofuran-2(3H)-one #; 2,3-dihydrobenzo[b]furan-2-one; DTXSID70203829; BENZO(B)FURAN-2(3H)-ONE; ACT10791; CS-M0764; ZINC1757785; BDBM50029069; OXAZOLE-2-CARBOTHIOICACIDAMIDE; AKOS009031219; NSC 227414; 2-coumaranone; (3h)-benzofuran-2-one; AS-18012; SY038967; DB-022573; C1445; FT-0612088; EN300-21214; A26517; L10016; J-509185; Q27259814; BENZENEACETIC ACID, 2-HYDROXY-, .GAMMA.-LACTONE; ACETIC ACID, (O-HYDROXYPHENYL)-, .GAMMA.-LACTONE
|
|
| CAS | 553-86-6 | |
| PubChem CID | 68382 | |
| ChEMBL ID | CHEMBL284584 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 134.13 | ALogp: | 1.3 |
| HBD: | 0 | HBA: | 2 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 26.3 | Aromatic Rings: | 2 |
| Heavy Atoms: | 10 | QED Weighted: | 0.398 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.594 | MDCK Permeability: | 0.00002220 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.255 |
| Human Intestinal Absorption (HIA): | 0.006 | 20% Bioavailability (F20%): | 0.888 |
| 30% Bioavailability (F30%): | 0.906 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.239 | Plasma Protein Binding (PPB): | 92.28% |
| Volume Distribution (VD): | 0.849 | Fu: | 10.17% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.914 | CYP1A2-substrate: | 0.716 |
| CYP2C19-inhibitor: | 0.156 | CYP2C19-substrate: | 0.091 |
| CYP2C9-inhibitor: | 0.044 | CYP2C9-substrate: | 0.776 |
| CYP2D6-inhibitor: | 0.161 | CYP2D6-substrate: | 0.774 |
| CYP3A4-inhibitor: | 0.018 | CYP3A4-substrate: | 0.215 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 11.401 | Half-life (T1/2): | 0.83 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.013 | Human Hepatotoxicity (H-HT): | 0.15 |
| Drug-inuced Liver Injury (DILI): | 0.582 | AMES Toxicity: | 0.266 |
| Rat Oral Acute Toxicity: | 0.422 | Maximum Recommended Daily Dose: | 0.032 |
| Skin Sensitization: | 0.391 | Carcinogencity: | 0.835 |
| Eye Corrosion: | 0.742 | Eye Irritation: | 0.991 |
| Respiratory Toxicity: | 0.638 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000038 | 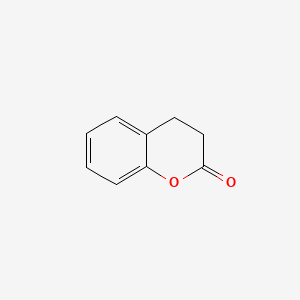 |
0.694 | D06DLI | 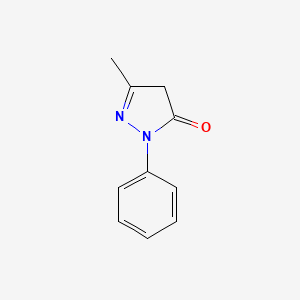 |
0.347 | ||
| ENC002236 |  |
0.500 | D0D5GG | 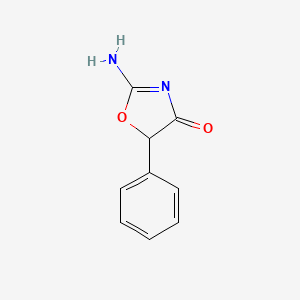 |
0.320 | ||
| ENC000171 |  |
0.479 | D06BYV |  |
0.308 | ||
| ENC000673 |  |
0.439 | D07HBX |  |
0.302 | ||
| ENC005244 |  |
0.432 | D03GET | 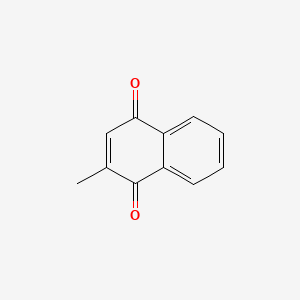 |
0.300 | ||
| ENC004792 | 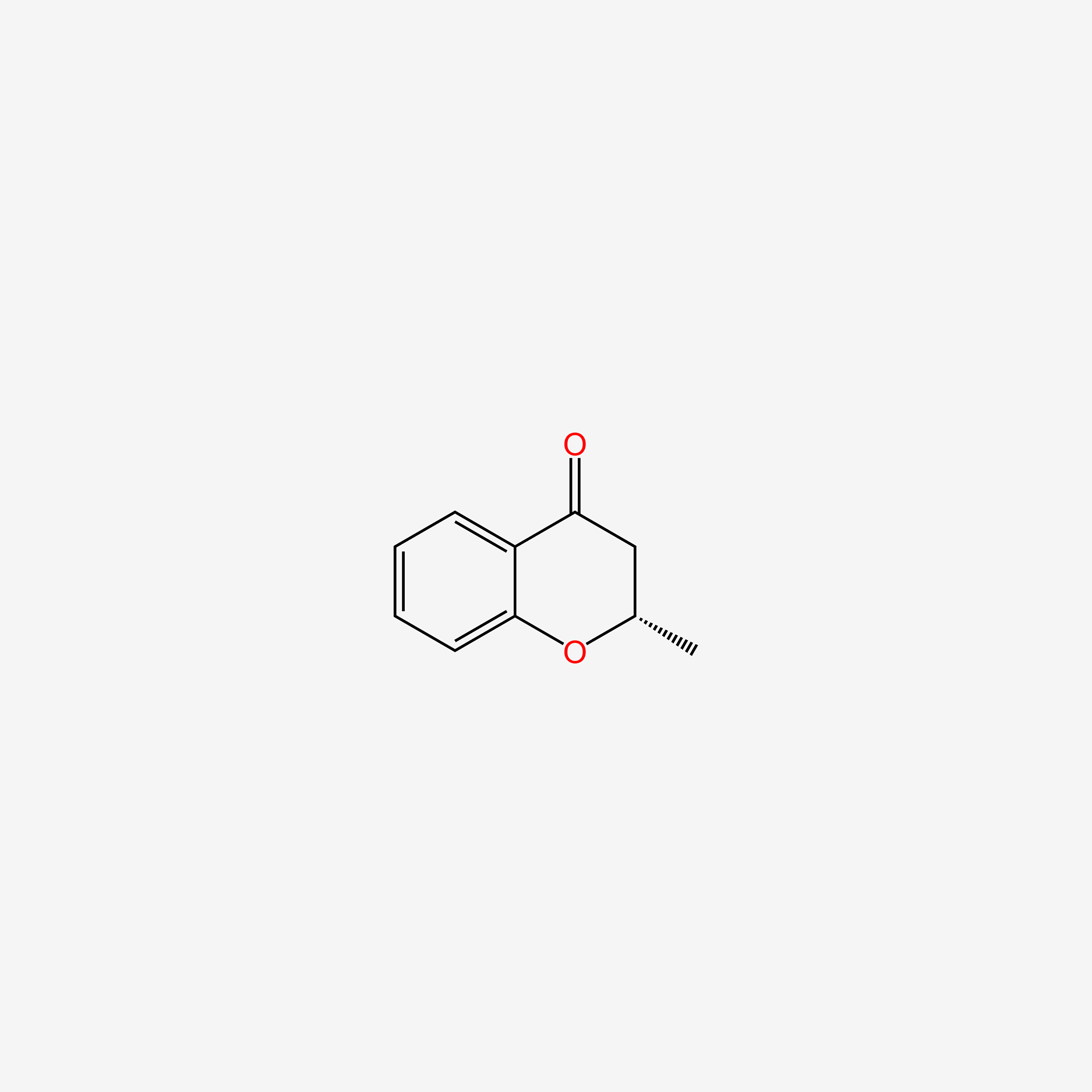 |
0.432 | D0QL3P | 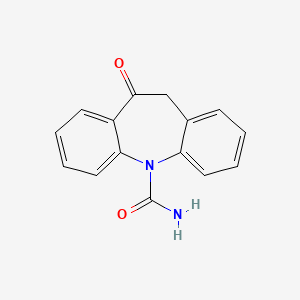 |
0.297 | ||
| ENC000345 | 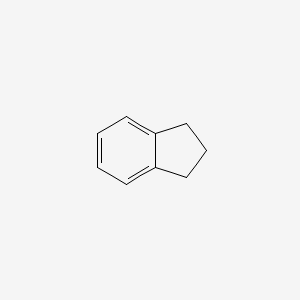 |
0.366 | D08EOD | 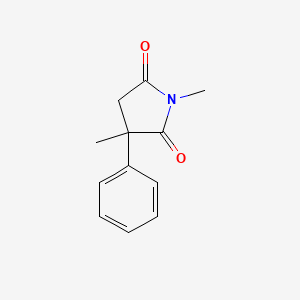 |
0.296 | ||
| ENC001031 | 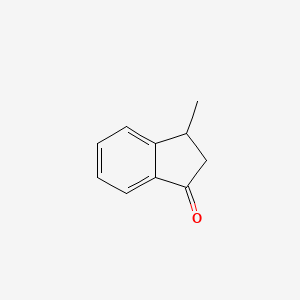 |
0.364 | D0R8PX | 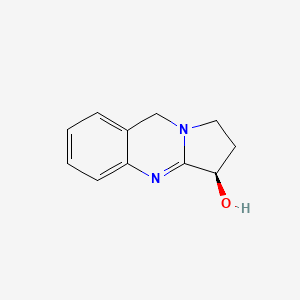 |
0.296 | ||
| ENC000025 | 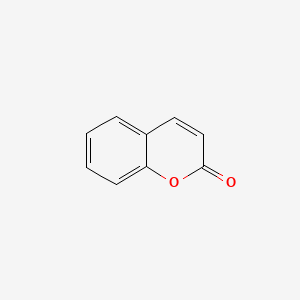 |
0.356 | D0MP5H | 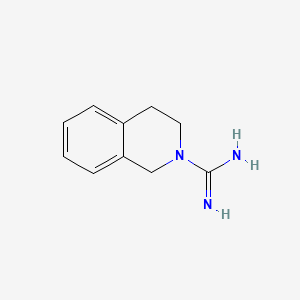 |
0.294 | ||
| ENC000675 | 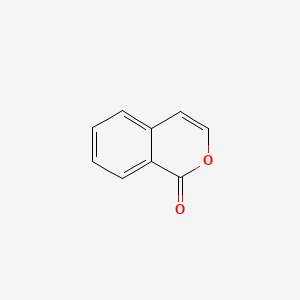 |
0.356 | D06OMW | 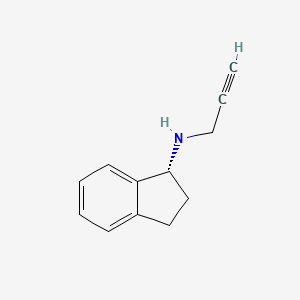 |
0.288 | ||