NPs Basic Information
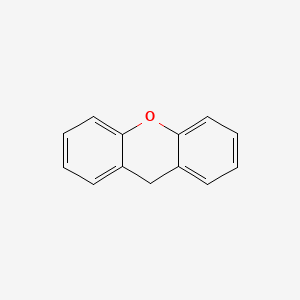
|
Name |
9H-Xanthene
|
| Molecular Formula | C13H10O | |
| IUPAC Name* |
9H-xanthene
|
|
| SMILES |
C1C2=CC=CC=C2OC3=CC=CC=C31
|
|
| InChI |
InChI=1S/C13H10O/c1-3-7-12-10(5-1)9-11-6-2-4-8-13(11)14-12/h1-8H,9H2
|
|
| InChIKey |
GJCOSYZMQJWQCA-UHFFFAOYSA-N
|
|
| Synonyms |
9H-Xanthene; XANTHENE; 92-83-1; 10H-9-Oxaanthracene; Xanthan; Dibenzo[a,e]pyran; NSC 46931; 9-oxa-9,10-dihydroanthracene; CHEBI:10057; NSC-46931; A762Z8101Y; Xanthenes; SMR000857210; EINECS 202-194-4; BRN 0133939; xanthene-; AI3-01544; UNII-A762Z8101Y; Xanthene, 99%; Dibenzopyran, tricyclic; SCHEMBL4267; 5-17-02-00252 (Beilstein Handbook Reference); MLS001333245; MLS001333246; CHEMBL486760; DTXSID1059070; HMS2231P24; HMS3371P15; ZINC967535; NSC46931; MFCD00005055; AKOS016008734; CS-W017106; NCGC00247062-01; AS-56278; 4-(ACETOXYMETHYL)BENZENEBORONICACID; DB-057333; FT-0603304; X0003; C01464; D70448; EN300-7407236; A859966; AE-562/43285774; Q413791; (R)-2-Piperazinecarboxylic acid dihydrochloride, 98%; Z1255415427
|
|
| CAS | 92-83-1 | |
| PubChem CID | 7107 | |
| ChEMBL ID | CHEMBL486760 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 182.22 | ALogp: | 3.5 |
| HBD: | 0 | HBA: | 1 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 9.2 | Aromatic Rings: | 3 |
| Heavy Atoms: | 14 | QED Weighted: | 0.507 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.709 | MDCK Permeability: | 0.00003000 |
| Pgp-inhibitor: | 0.013 | Pgp-substrate: | 0.034 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.862 |
| 30% Bioavailability (F30%): | 0.008 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.628 | Plasma Protein Binding (PPB): | 97.59% |
| Volume Distribution (VD): | 0.841 | Fu: | 2.83% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.955 | CYP1A2-substrate: | 0.597 |
| CYP2C19-inhibitor: | 0.943 | CYP2C19-substrate: | 0.2 |
| CYP2C9-inhibitor: | 0.582 | CYP2C9-substrate: | 0.864 |
| CYP2D6-inhibitor: | 0.106 | CYP2D6-substrate: | 0.75 |
| CYP3A4-inhibitor: | 0.11 | CYP3A4-substrate: | 0.566 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 11.166 | Half-life (T1/2): | 0.565 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.04 | Human Hepatotoxicity (H-HT): | 0.043 |
| Drug-inuced Liver Injury (DILI): | 0.727 | AMES Toxicity: | 0.359 |
| Rat Oral Acute Toxicity: | 0.032 | Maximum Recommended Daily Dose: | 0.21 |
| Skin Sensitization: | 0.713 | Carcinogencity: | 0.628 |
| Eye Corrosion: | 0.011 | Eye Irritation: | 0.966 |
| Respiratory Toxicity: | 0.373 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000159 | 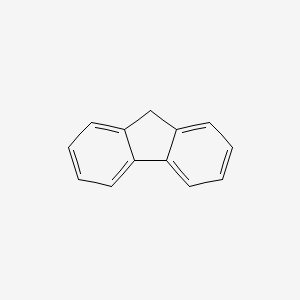 |
0.620 | D0DV3O | 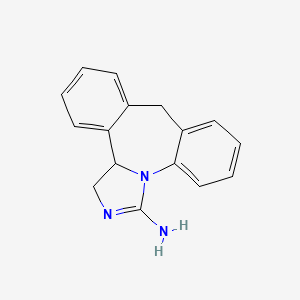 |
0.463 | ||
| ENC000681 | 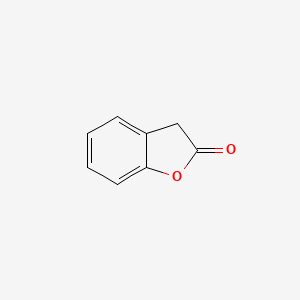 |
0.479 | D0R6RO | 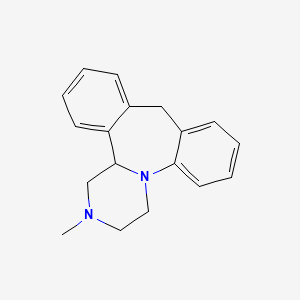 |
0.443 | ||
| ENC000036 | 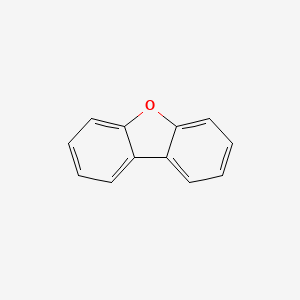 |
0.446 | D0QL3P | 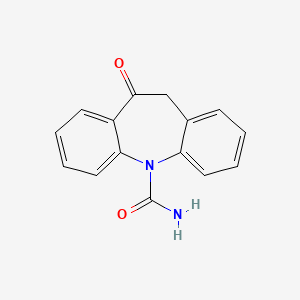 |
0.412 | ||
| ENC000047 | 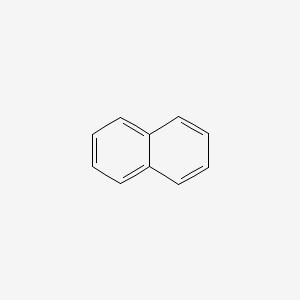 |
0.440 | D06FES | 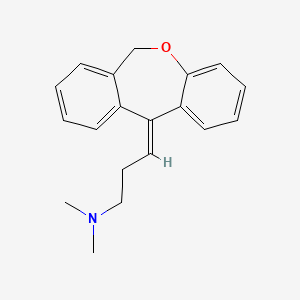 |
0.411 | ||
| ENC000737 | 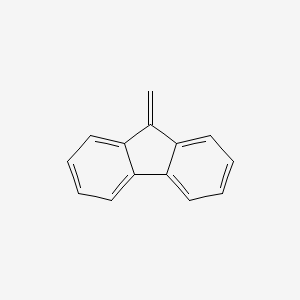 |
0.407 | D04WFD | 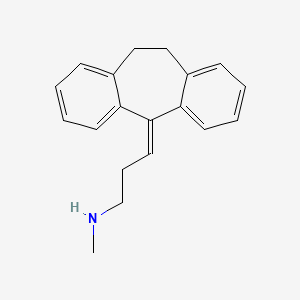 |
0.403 | ||
| ENC000038 | 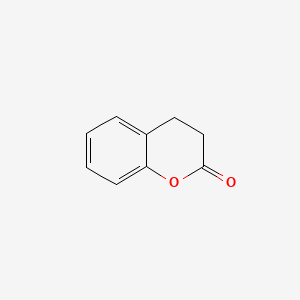 |
0.396 | D01UTL |  |
0.403 | ||
| ENC000321 |  |
0.385 | D06ZUK |  |
0.392 | ||
| ENC001050 |  |
0.377 | D0Y5UG |  |
0.392 | ||
| ENC003440 | 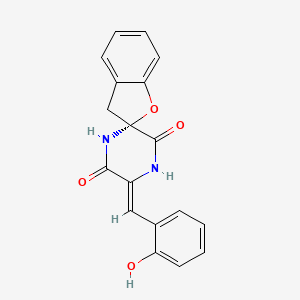 |
0.370 | D04QZD | 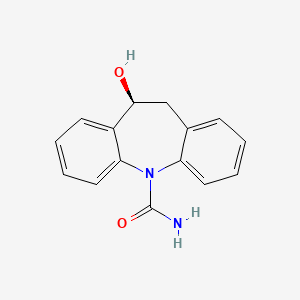 |
0.391 | ||
| ENC000732 | 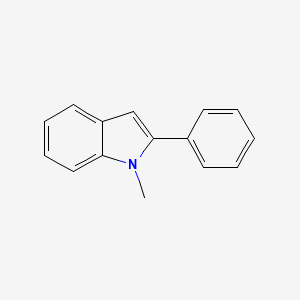 |
0.369 | D00HZV | 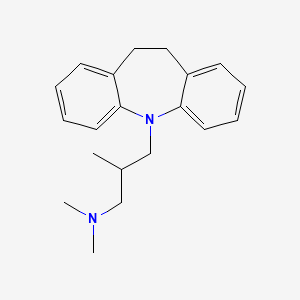 |
0.382 | ||