NPs Basic Information
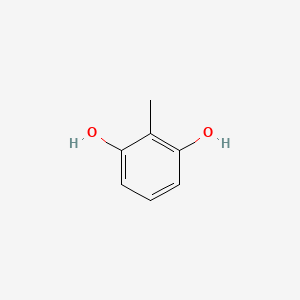
|
Name |
2-Methylresorcinol
|
| Molecular Formula | C7H8O2 | |
| IUPAC Name* |
2-methylbenzene-1,3-diol
|
|
| SMILES |
CC1=C(C=CC=C1O)O
|
|
| InChI |
InChI=1S/C7H8O2/c1-5-6(8)3-2-4-7(5)9/h2-4,8-9H,1H3
|
|
| InChIKey |
ZTMADXFOCUXMJE-UHFFFAOYSA-N
|
|
| Synonyms |
2-Methylresorcinol; 608-25-3; 2-methylbenzene-1,3-diol; 2,6-Dihydroxytoluene; 1,3-Benzenediol, 2-methyl-; 2-Methylresorcin; 2-Methyl-1,3-benzenediol; Resorcinol, 2-methyl-; Toluene-2,6-diol; 1,3-Benzenediol, methyl-; 1,3-DIHYDROXY-2-METHYLBENZENE; 2-Methyl-1,3-dihydroxybenzene; 2-METHYL RESORCINOL; MFCD00002271; 2-methyl-benzene-1,3-diol; CHEMBL2332773; NSC-66524; 32W7044A3T; EINECS 210-155-8; NSC 66524; BRN 2042177; AI3-61050; methyl resorcinol; UNII-32W7044A3T; 2-Methyl-resorcinol; ORISTAR MR; RODOL MRS; 2,6-Dihydroxy-toluene; 3-hydroxy-2-methylphenol; DSSTox_CID_5571; 2-Methylresorcinol, 98%; EC 210-155-8; DSSTox_RID_77835; DSSTox_GSID_25571; SCHEMBL33721; 4-06-00-05877 (Beilstein Handbook Reference); 2-Methyl-1,3-benzenediol #; 2,6-dihydroxy-1-methylbenzene; 2-MR; DTXSID0025571; 2-METHYLRESORCINOL [INCI]; ZINC154621; ACT07472; AMY19648; NSC66524; STR04303; Tox21_301973; BDBM50187503; STL183298; AKOS000121503; METHYL-1,3-BENZENEDIOL, 2-; CS-W013532; EG-0038; NCGC00255355-01; AC-11239; CAS-608-25-3; 2-Methylresorcinol, technical grade, 90%; DB-053715; FT-0613080; FT-0654680; M0425; M1109; 2-Methylresorcinol, technical, >=90% (GC); EN300-16605; D70597; M-4340; A832909; Q9549707; F0001-1592; 1251900-91-0
|
|
| CAS | 608-25-3 | |
| PubChem CID | 11843 | |
| ChEMBL ID | CHEMBL2332773 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 124.14 | ALogp: | 1.6 |
| HBD: | 2 | HBA: | 2 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 40.5 | Aromatic Rings: | 1 |
| Heavy Atoms: | 9 | QED Weighted: | 0.554 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.473 | MDCK Permeability: | 0.00002140 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.016 |
| Human Intestinal Absorption (HIA): | 0.006 | 20% Bioavailability (F20%): | 0.622 |
| 30% Bioavailability (F30%): | 0.496 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.094 | Plasma Protein Binding (PPB): | 75.38% |
| Volume Distribution (VD): | 0.793 | Fu: | 16.32% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.709 | CYP1A2-substrate: | 0.901 |
| CYP2C19-inhibitor: | 0.198 | CYP2C19-substrate: | 0.17 |
| CYP2C9-inhibitor: | 0.126 | CYP2C9-substrate: | 0.859 |
| CYP2D6-inhibitor: | 0.479 | CYP2D6-substrate: | 0.763 |
| CYP3A4-inhibitor: | 0.092 | CYP3A4-substrate: | 0.224 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 16.204 | Half-life (T1/2): | 0.917 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.009 | Human Hepatotoxicity (H-HT): | 0.031 |
| Drug-inuced Liver Injury (DILI): | 0.047 | AMES Toxicity: | 0.3 |
| Rat Oral Acute Toxicity: | 0.905 | Maximum Recommended Daily Dose: | 0.105 |
| Skin Sensitization: | 0.922 | Carcinogencity: | 0.535 |
| Eye Corrosion: | 0.988 | Eye Irritation: | 0.983 |
| Respiratory Toxicity: | 0.743 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000060 | 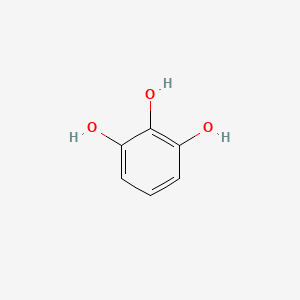 |
0.600 | D0T7OW | 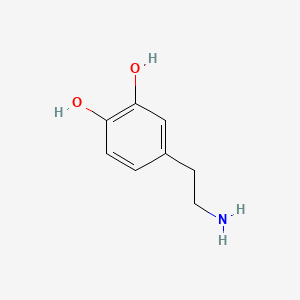 |
0.350 | ||
| ENC000690 | 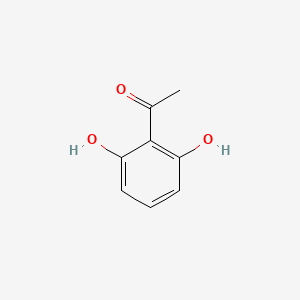 |
0.559 | D07HBX |  |
0.342 | ||
| ENC000021 | 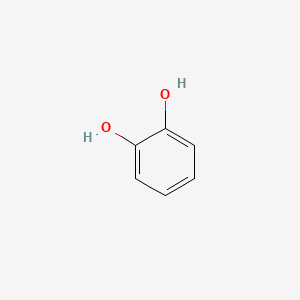 |
0.533 | D04PHC | 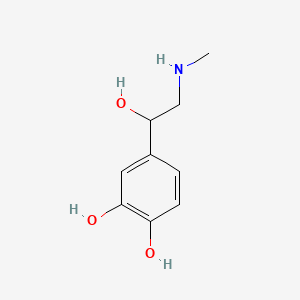 |
0.341 | ||
| ENC000329 | 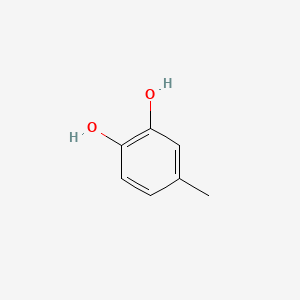 |
0.500 | D07MOX | 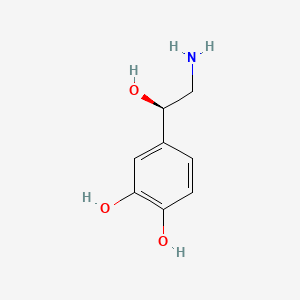 |
0.333 | ||
| ENC001513 | 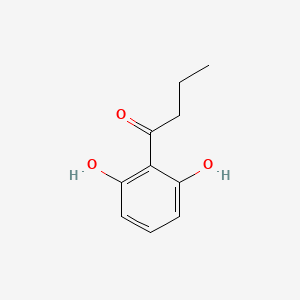 |
0.475 | D06GIP | 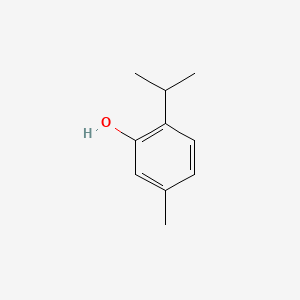 |
0.325 | ||
| ENC000390 | 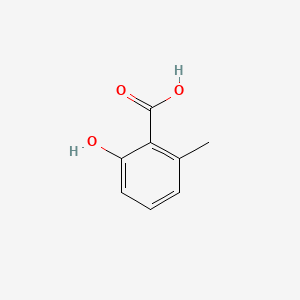 |
0.472 | D03UOT |  |
0.314 | ||
| ENC002350 | 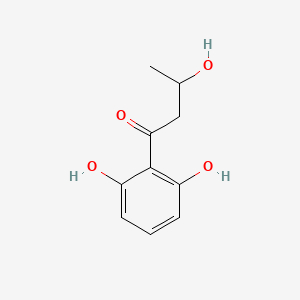 |
0.452 | D0BA6T | 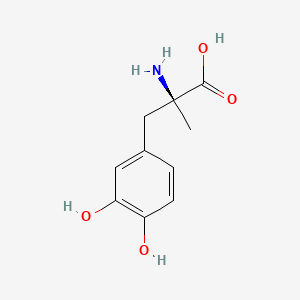 |
0.313 | ||
| ENC000683 | 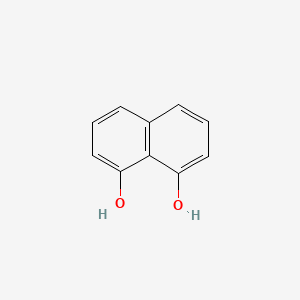 |
0.450 | D0V9EN |  |
0.311 | ||
| ENC002237 | 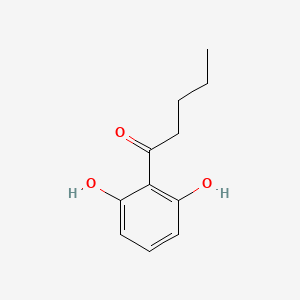 |
0.442 | D0I8FI | 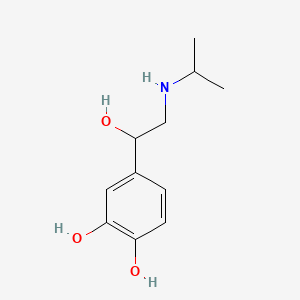 |
0.306 | ||
| ENC000028 | 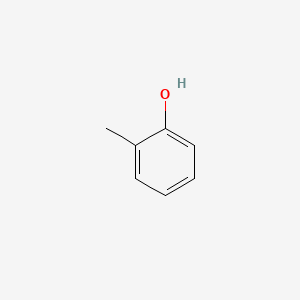 |
0.438 | D0U0OT | 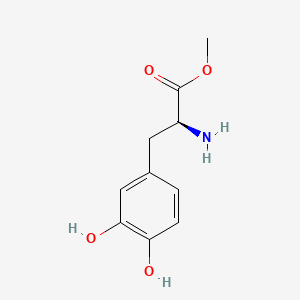 |
0.306 | ||