NPs Basic Information
|
Name |
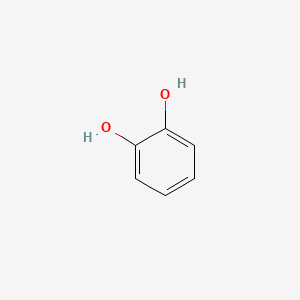
Catechol
|
| Molecular Formula | C6H6O2 | |
| IUPAC Name* |
benzene-1,2-diol
|
|
| SMILES |
C1=CC=C(C(=C1)O)O
|
|
| InChI |
InChI=1S/C6H6O2/c7-5-3-1-2-4-6(5)8/h1-4,7-8H
|
|
| InChIKey |
YCIMNLLNPGFGHC-UHFFFAOYSA-N
|
|
| Synonyms |
pyrocatechol; catechol; 120-80-9; 1,2-benzenediol; 1,2-dihydroxybenzene; benzene-1,2-diol; pyrocatechin; 2-hydroxyphenol; o-Benzenediol; o-Dihydroxybenzene; o-Dioxybenzene; o-Hydroquinone; o-Hydroxyphenol; Phthalhydroquinone; Pyrocatechine; o-Phenylenediol; Oxyphenic acid; Fouramine PCH; benzenediol; Durafur developer C; Pelagol Grey C; Catechin (phenol); Fourrine 68; Benzene, o-dihydroxy-; Catechol (phenol); o-Diphenol; C.I. Oxidation Base 26; Pyrokatechin; Pyrokatechol; Katechol; ortho-Dihydroxybenzene; NCI-C55856; NSC 1573; C.I. 76500; Catechol-pyrocatechol; 12385-08-9; MFCD00002188; LF3AJ089DQ; CHEMBL280998; DTXSID3020257; CHEBI:18135; NSC-1573; ortho-Hydroxyphenol; 26982-53-6; CAQ; DSSTox_CID_257; ortho-Benzenediol; ortho-Dioxybenzene; ortho-Hydroquinone; DSSTox_RID_75468; Katechol [Czech]; ortho-Phenylenediol; Pyrocatechinic acid; DSSTox_GSID_20257; Pyrokatechin [Czech]; Pyrokatechol [Czech]; Benzene-1,2-diol (Pyrocatechol); CI Oxidation Base 26; Phthalic alcohol; CAS-120-80-9; SMR000326660; CCRIS 741; HSDB 1436; EINECS 204-427-5; UNII-LF3AJ089DQ; BRN 0471401; Oxyphenate; CI 76500; Kachin; ortho-diphenol; benzene diol; ortho-Quinol; AI3-03995; 4oow; alpha-hydroxyphenol; 1,2-benzenedio; o-dihydroxy-benzene; phenol derivative, 2; 3fw4; 4k7i; CATECHOL [HSDB]; CATECHOL [IARC]; Pyrocatechol, >=99%; CATECHOL [VANDF]; Lopac-C-9510; PYROCATECHOL [MI]; WLN: QR BQ; bmse000385; EC 204-427-5; PYROCATECHOL [INCI]; 1,2-Dihydroxybenzene, XI; 1,2-Benzenediol; Catechol; Lopac0_000280; SCHEMBL18351; MLS002153385; MLS002303022; BIDD:ER0327; Pyrocatechinic acidPyrocatechol; Pyrocatechol, p.a., 99.0%; BDBM26188; Durafur Developer CFouramine PCH; NSC1573; HMS2233A17; HMS3260H22; HMS3373K16; Tox21_202317; Tox21_300153; Tox21_500280; s6305; STK398651; ZINC13512214; AKOS000119002; CCG-204375; DB02232; LP00280; SDCCGSBI-0050268.P002; NCGC00015283-01; NCGC00015283-02; NCGC00015283-03; NCGC00015283-04; NCGC00015283-05; NCGC00015283-06; NCGC00015283-07; NCGC00015283-08; NCGC00015283-10; NCGC00091262-01; NCGC00091262-02; NCGC00091262-03; NCGC00253952-01; NCGC00259866-01; NCGC00260965-01; AC-34196; BP-21156; BS-20054; Catechol (Pyrocatechol; Benzene-1,2-diol); DB-003770; C.I.-76500; EU-0100280; FT-0606411; P0317; P0567; EN300-19426; C 9510; C00090; C01785; D91943; 1,2-Dihydroxybenzene, ReagentPlus(R), >=99%; Pyrocatechol, purified by sublimation, >=99.5%; A804599; AB-131/40235236; Q282440; SR-01000075791; SR-01000075791-1; W-109068; F0001-0332; Pyrocatechol, certified reference material, TraceCERT(R); Z104473810; Pyrocatechol, plant cell culture tested, BioReagent, >=99%, powder; 2H-1-Benzopyran-3,5,7-triol, 2-(3,4-dihydroxyphenyl)-3,4-dihydro-,(2R-trans)-
|
|
| CAS | 120-80-9 | |
| PubChem CID | 289 | |
| ChEMBL ID | CHEMBL280998 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 110.11 | ALogp: | 0.9 |
| HBD: | 2 | HBA: | 2 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 40.5 | Aromatic Rings: | 1 |
| Heavy Atoms: | 8 | QED Weighted: | 0.497 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.444 | MDCK Permeability: | 0.00002120 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.003 |
| Human Intestinal Absorption (HIA): | 0.016 | 20% Bioavailability (F20%): | 0.957 |
| 30% Bioavailability (F30%): | 0.799 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.083 | Plasma Protein Binding (PPB): | 56.25% |
| Volume Distribution (VD): | 0.681 | Fu: | 29.84% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.354 | CYP1A2-substrate: | 0.398 |
| CYP2C19-inhibitor: | 0.105 | CYP2C19-substrate: | 0.093 |
| CYP2C9-inhibitor: | 0.058 | CYP2C9-substrate: | 0.664 |
| CYP2D6-inhibitor: | 0.213 | CYP2D6-substrate: | 0.648 |
| CYP3A4-inhibitor: | 0.027 | CYP3A4-substrate: | 0.201 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 19.007 | Half-life (T1/2): | 0.93 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.005 | Human Hepatotoxicity (H-HT): | 0.035 |
| Drug-inuced Liver Injury (DILI): | 0.058 | AMES Toxicity: | 0.62 |
| Rat Oral Acute Toxicity: | 0.898 | Maximum Recommended Daily Dose: | 0.022 |
| Skin Sensitization: | 0.933 | Carcinogencity: | 0.726 |
| Eye Corrosion: | 0.983 | Eye Irritation: | 0.991 |
| Respiratory Toxicity: | 0.906 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000028 | 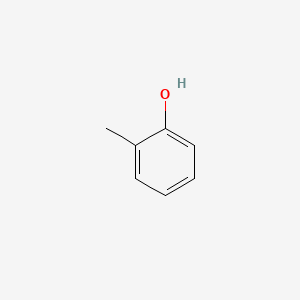 |
0.571 | D07HBX |  |
0.531 | ||
| ENC000404 | 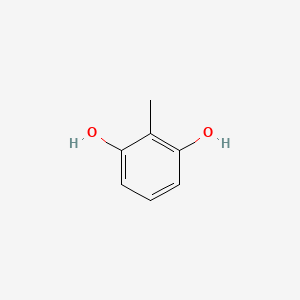 |
0.533 | D03UOT |  |
0.375 | ||
| ENC000060 | 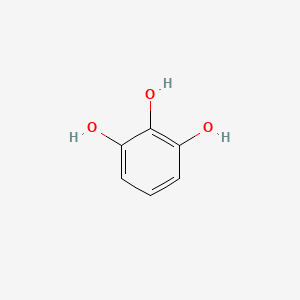 |
0.533 | D0T7OW | 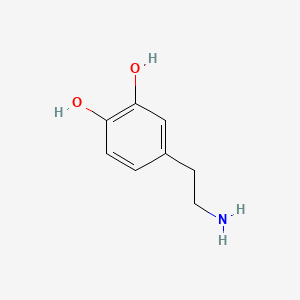 |
0.368 | ||
| ENC000033 | 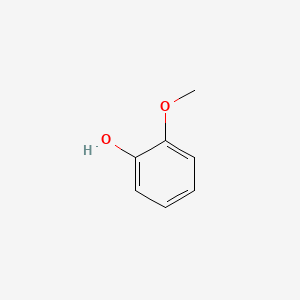 |
0.516 | D05OIS |  |
0.364 | ||
| ENC000166 | 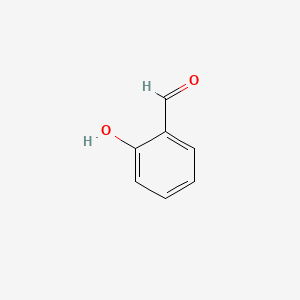 |
0.516 | D0F5ZM | 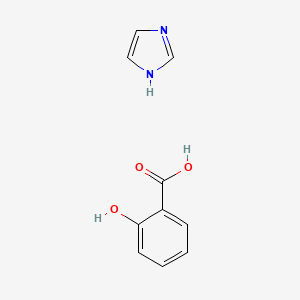 |
0.362 | ||
| ENC000754 | 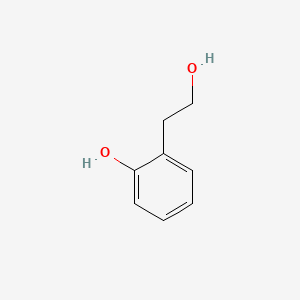 |
0.515 | D07MOX | 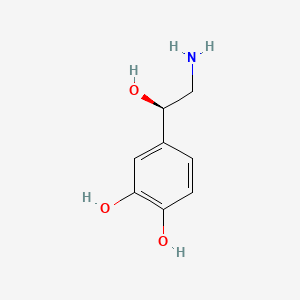 |
0.350 | ||
| ENC005498 | 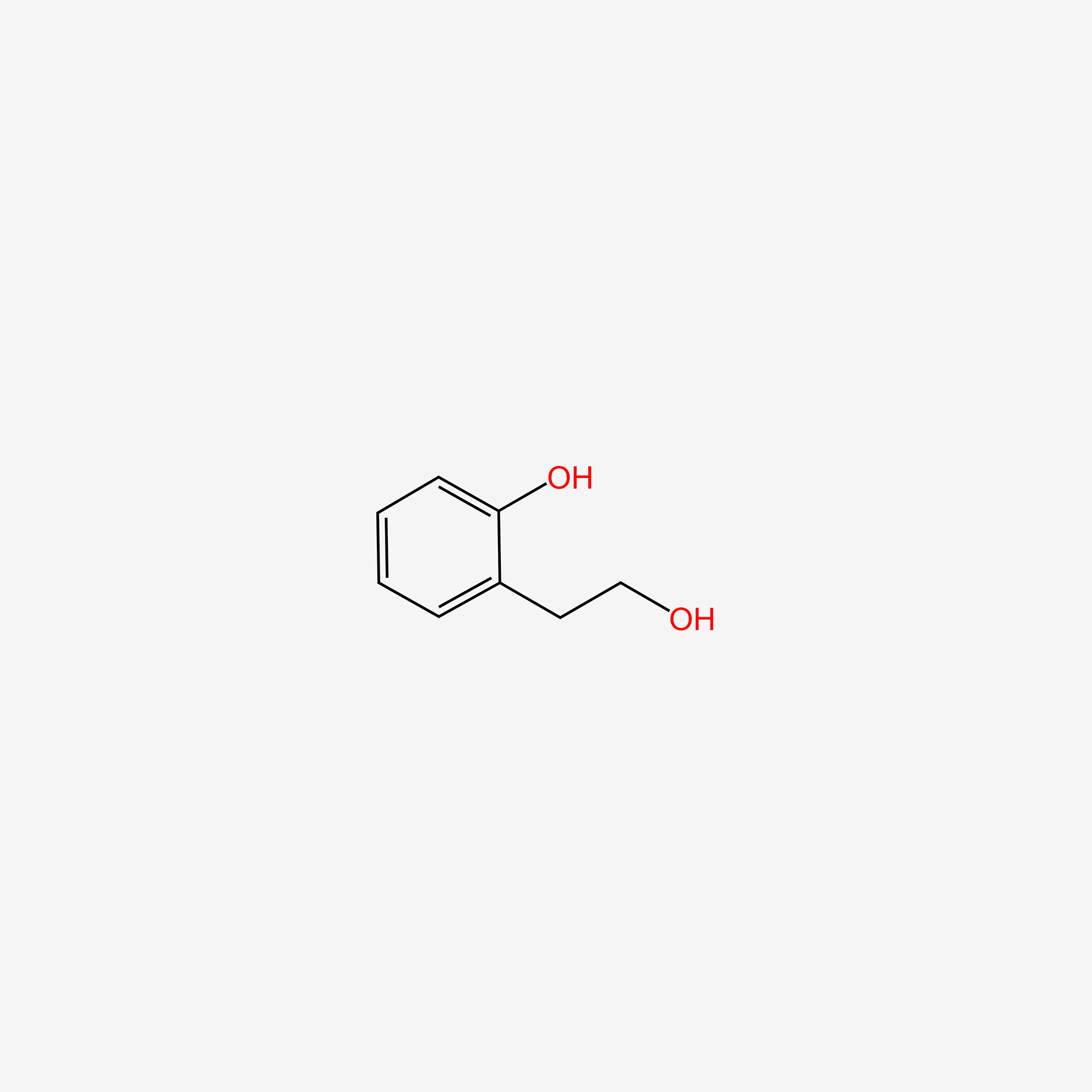 |
0.515 | D0V9EN |  |
0.326 | ||
| ENC000409 | 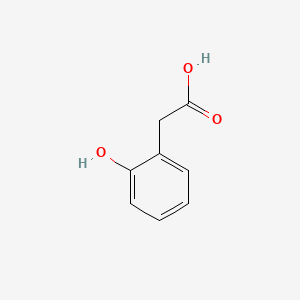 |
0.486 | D04PHC | 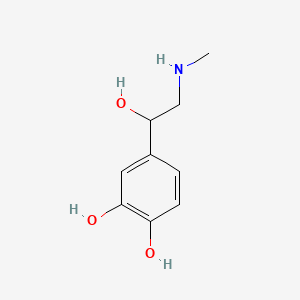 |
0.326 | ||
| ENC000108 | 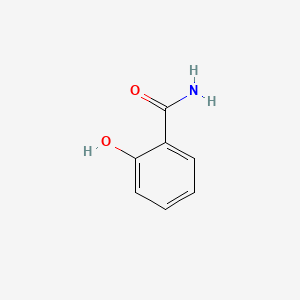 |
0.485 | D0N3UL | 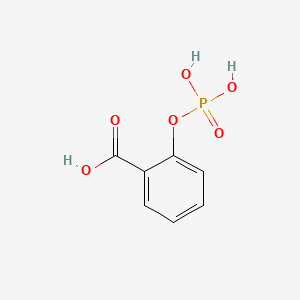 |
0.311 | ||
| ENC000683 | 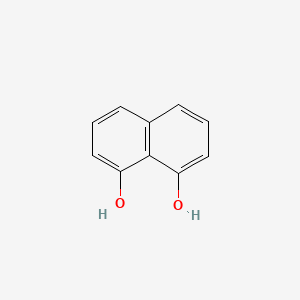 |
0.474 | D08HVR | 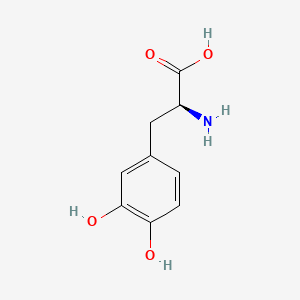 |
0.311 | ||