NPs Basic Information
|
Name |
Hexadecane
|
| Molecular Formula | C16H34 | |
| IUPAC Name* |
hexadecane
|
|
| SMILES |
CCCCCCCCCCCCCCCC
|
|
| InChI |
InChI=1S/C16H34/c1-3-5-7-9-11-13-15-16-14-12-10-8-6-4-2/h3-16H2,1-2H3
|
|
| InChIKey |
DCAYPVUWAIABOU-UHFFFAOYSA-N
|
|
| Synonyms |
HEXADECANE; n-Hexadecane; 544-76-3; Cetane; n-Cetane; hexadecan; Hexadekan; Pentadecane, methyl-; Cetan; CCRIS 5833; Zetan; HSDB 6854; NSC 7334; EINECS 208-878-9; F8Z00SHP6Q; BRN 1736592; AI3-06522; MFCD00008998; CHEBI:45296; NSC-7334; Hexadecane, analytical standard; CNS; UNII-F8Z00SHP6Q; Hexadecane, >=99%; HEXADECANE, N-; HEXADECANE [HSDB]; HEXADECANE [INCI]; DSSTox_CID_7195; EC 208-878-9; Hexadecane, p.a., 99%; DSSTox_RID_78343; DSSTox_GSID_27195; 4-01-00-00537 (Beilstein Handbook Reference); Hexadecane_RamanathanGurudeeban; PARAFOL 16-97; CHEMBL134994; QSPL 025; QSPL 078; QSPL 116; DTXSID0027195; Hexadecane, anhydrous, >=99%; NSC7334; CH3-[CH2]14-CH3; Hexadecane, ReagentPlus(R), 99%; Tox21_300485; LMFA11000577; STL453674; ZINC38141452; AKOS025212855; n-Hexadecane 10 microg/mL in Acetone; Hexadecane, purum, >=98.0% (GC); NCGC00164132-01; NCGC00164132-02; NCGC00254306-01; AS-56424; CAS-544-76-3; DB-052582; Hexadecane, Vetec(TM) reagent grade, 98%; CS-0152222; FT-0632360; H0066; S0288; D97389; A830206; Q150843; 5166841B-BF92-4A7D-8CEF-0B01B374ED0E
|
|
| CAS | 544-76-3 | |
| PubChem CID | 11006 | |
| ChEMBL ID | CHEMBL134994 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 226.44 | ALogp: | 8.3 |
| HBD: | 0 | HBA: | 0 |
| Rotatable Bonds: | 13 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 0.0 | Aromatic Rings: | 0 |
| Heavy Atoms: | 16 | QED Weighted: | 0.317 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.767 | MDCK Permeability: | 0.00000864 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.002 | 20% Bioavailability (F20%): | 0.347 |
| 30% Bioavailability (F30%): | 0.998 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.112 | Plasma Protein Binding (PPB): | 98.31% |
| Volume Distribution (VD): | 3.848 | Fu: | 1.58% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.307 | CYP1A2-substrate: | 0.192 |
| CYP2C19-inhibitor: | 0.393 | CYP2C19-substrate: | 0.075 |
| CYP2C9-inhibitor: | 0.108 | CYP2C9-substrate: | 0.946 |
| CYP2D6-inhibitor: | 0.247 | CYP2D6-substrate: | 0.059 |
| CYP3A4-inhibitor: | 0.194 | CYP3A4-substrate: | 0.047 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 4.564 | Half-life (T1/2): | 0.069 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.233 | Human Hepatotoxicity (H-HT): | 0.008 |
| Drug-inuced Liver Injury (DILI): | 0.242 | AMES Toxicity: | 0.007 |
| Rat Oral Acute Toxicity: | 0.035 | Maximum Recommended Daily Dose: | 0.03 |
| Skin Sensitization: | 0.951 | Carcinogencity: | 0.036 |
| Eye Corrosion: | 0.995 | Eye Irritation: | 0.939 |
| Respiratory Toxicity: | 0.51 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000427 |  |
0.939 | D07ILQ |  |
0.631 | ||
| ENC000423 |  |
0.935 | D0Z5SM |  |
0.613 | ||
| ENC000400 |  |
0.885 | D00AOJ |  |
0.569 | ||
| ENC000422 |  |
0.870 | D00FGR |  |
0.532 | ||
| ENC000428 |  |
0.836 | D05ATI |  |
0.525 | ||
| ENC000421 |  |
0.804 | D0O1PH |  |
0.455 | ||
| ENC000426 |  |
0.804 | D0T9TJ |  |
0.412 | ||
| ENC000285 |  |
0.793 | D00MLW |  |
0.383 | ||
| ENC000489 | 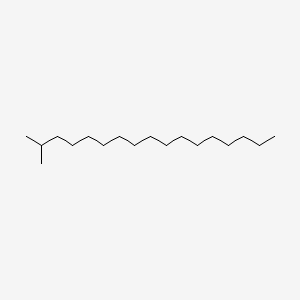 |
0.764 | D05QNO | 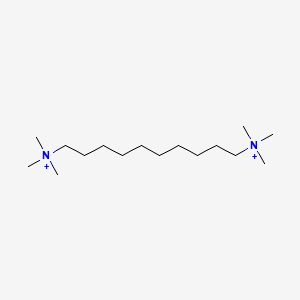 |
0.382 | ||
| ENC000557 | 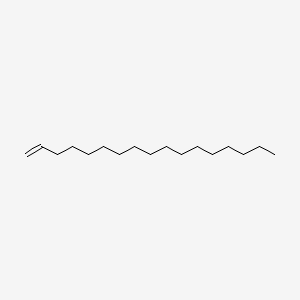 |
0.759 | D0P1RL | 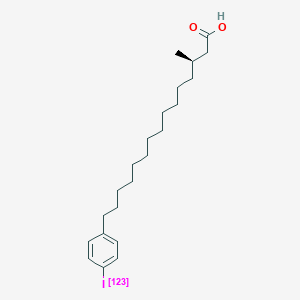 |
0.376 | ||